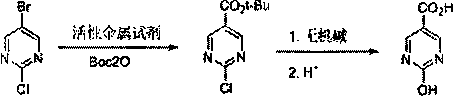
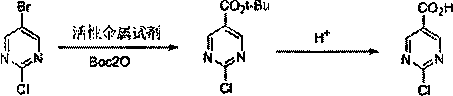
The synthetic method of 2-chloro/hydroxypyrimidine-5-carboxylic acid
A synthesis method and chloropyrimidine technology are applied in the fields of organic chemistry, products, reagents, etc., which can solve problems such as low yield and achieve the effect of good operation reproducibility.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Under nitrogen protection, add 2-chloro-5-bromopyrimidine (19.3 g, 0.1 mol) and THF35mL into a three-necked reaction flask, then cool down to -10°C, and start to dropwise add 1.3M isopropylmagnesium chloride-lithium chloride tetrahydrofuran solution (100 mL, 0.13 mol). After the dropwise addition was completed, the reaction was continued for 1.5 hours, and then a solution of Boc2O (24.0 g, 0.11 mol) dissolved in THF (25 mL) was started to be added dropwise.
[0027] After the reaction is completed by sampling and detection, add 10% hydrochloric acid to quench to neutrality, then add 36% hydrochloric acid to adjust to pH1 HNMR(400MHz, DMSO-d6): 13.66(s, 1H), 9.14(s, 2H).
Embodiment 2
[0029] Under the protection of nitrogen, add 2-chloro-5-bromopyrimidine (19.3 g, 0.1 mol) and 35 mL of 2-MeTHF into the three-necked reaction flask, then lower the temperature to -70°C to -75°C, and start adding 2.5M n-butyllithium dropwise / hexane solution (48mL, 0.12mol). After the dropwise addition was completed, the reaction was continued for 1 hour, and then a solution of Boc2O (24.0 g, 0.11 mol) dissolved in 2-MeTHF (25 mL) was added dropwise. .
[0030] After the reaction is finished, add 10% hydrochloric acid to quench to neutrality, then add 36% hydrochloric acid to adjust to pH<1, stir at room temperature for 3-4 hours, then add 1M sodium hydroxide to adjust pH=1-2, separate organic layer, washed twice with saturated brine, and dried over anhydrous magnesium sulfate. The solvent was evaporated to dryness by filtration, mixed solvent of dichloromethane / toluene was added for beating, and then vacuum-dried to obtain 13.0 g of light yellow solid 2-chloropyrimidine-5-ca...
Embodiment 3
[0032] Under nitrogen protection, 2-chloro-5-bromopyrimidine (19.3 g, 0.1 mol) and THF35mL were added to a three-necked reaction flask, then the temperature was lowered to 0°C, and 1M n-Bu3MgLi tetrahydrofuran solution (0.35mol) was added dropwise. After the dropwise addition was completed, the reaction was continued for 2.5 hours, and then a solution of Boc2O (24.0 g, 0.11 mol) dissolved in THF (25 mL) was added dropwise.
[0033] After the reaction was completed, 10% hydrochloric acid was added to quench to neutrality, then 36% hydrochloric acid was added to adjust to pH<1, stirred at room temperature for 4 hours, then 1M potassium hydroxide was added to adjust pH=2-3, the layers were separated, and two Extract twice with 80 mL of methyl chloride, combine the organic layers, wash with saturated brine, and dry over anhydrous magnesium sulfate. The solvent was evaporated to dryness by filtration, mixed solvent of dichloromethane / toluene was added for beating, and then vacuum-d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com