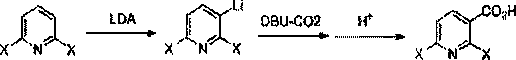Synthetic method of 2,6-dihalopyridine-3-carboxylic acid
A technique for the synthesis of dihalopyridines and synthesis methods, which is applied in the synthesis of pyridine compounds and the field of synthesis of 2,6-dihalopyridine-3-carboxylic acid, which can solve the problems of low yield and achieve good operation reproducibility Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Under the protection of nitrogen, 2,6-difluoropyridine (11.5 g, 0.1 mol), Boc2O (26.0 g, 0.12 mol) and 130 mL of tetrahydrofuran were stirred until completely dissolved, and the ice-salt bath was lowered to -15 ° C, and the drop Add a pre-prepared 1M LDA tetrahydrofuran solution (0.13 mol), and control the temperature not to exceed -10°C during the entire dropwise addition. After the dropwise addition, keep stirring for 2-3 hours, then naturally rise to room temperature and stir to react overnight, and TLC detects that there is basically no remaining raw material.
[0027] Lower the reaction solution to 0°C again, add 15% aqueous hydrochloric acid dropwise to pH = 1-2 (note that the acidity adjustment should not be too strong, when the acidic hydrolysis is not controlled, a small amount of 2-hydroxy-6-fluoro-pyridine- 3-carboxylic acid). During the dropwise addition process, heat is obviously released, control the temperature not to exceed 30°C, raise the temperature t...
Embodiment 2
[0029] Under the protection of nitrogen, 2,6-dichloropyridine (14.8 g, 0.1 mol), Boc2O (26.0 g, 0.12 mol) and 130 mL of tetrahydrofuran were stirred until completely dissolved, and the ice-salt bath was lowered to -15 ° C, and the drop Add the isopropylmagnesium chloride-lithium chloride solution dropwise into the diisopropylamine tetrahydrofuran solution in advance, and prepare a 1M diisopropylamine magnesium chloride-lithium chloride tetrahydrofuran solution (0.15 moles). over -10°C. After the dropwise addition, keep stirring for 2-3 hours, then naturally rise to room temperature and stir to react overnight, and TLC detects that there is basically no remaining raw material.
[0030] The reaction solution was lowered to 0°C again, and 36% aqueous hydrochloric acid was added dropwise until pH=1. During the dropwise addition process, the heat is obviously exothermic, and the temperature should not exceed 30°C. After the dropwise addition, the temperature should be raised to 35...
Embodiment 3
[0032] Under the protection of nitrogen, stir 2,6-difluoropyridine (11.5 g, 0.1 mol) and 120 mL of tetrahydrofuran until completely dissolved, then cool down the dry ice / acetone system to -75°C, and start to add the pre-prepared 1M LDA tetrahydrofuran dropwise solution (0.12 moles), the temperature control during the whole dropping process should not exceed -60°C. After the dropwise addition, keep stirring for 1.5 hours, then dissolve the DBU-CO2 complex (0.11mol) in 65mL tetrahydrofuran solution, control the temperature not to exceed -60°C and drop into the reaction solution, keep stirring for 2 hours, and naturally rise to The reaction was stirred at room temperature overnight, and there was almost no remaining raw material as detected by TLC.
[0033] The reaction solution was lowered to 0°C again, and 15% hydrochloric acid aqueous solution was added dropwise to pH=3-5. During the dropwise addition process, the heat is obviously released, and the temperature is controlled ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com