Chimeric antigen receptor DAP12-T2A-CD8alpha-MSLN scFv-NKp44 and purpose thereof
A technology of DAP12-T2A-CD8, -mslnscfv-nkp44, applied in the field of immune cells, can solve the problem of cytokine release syndrome without observed acute adverse events
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Embodiment 1, chimeric antigen receptor preparation
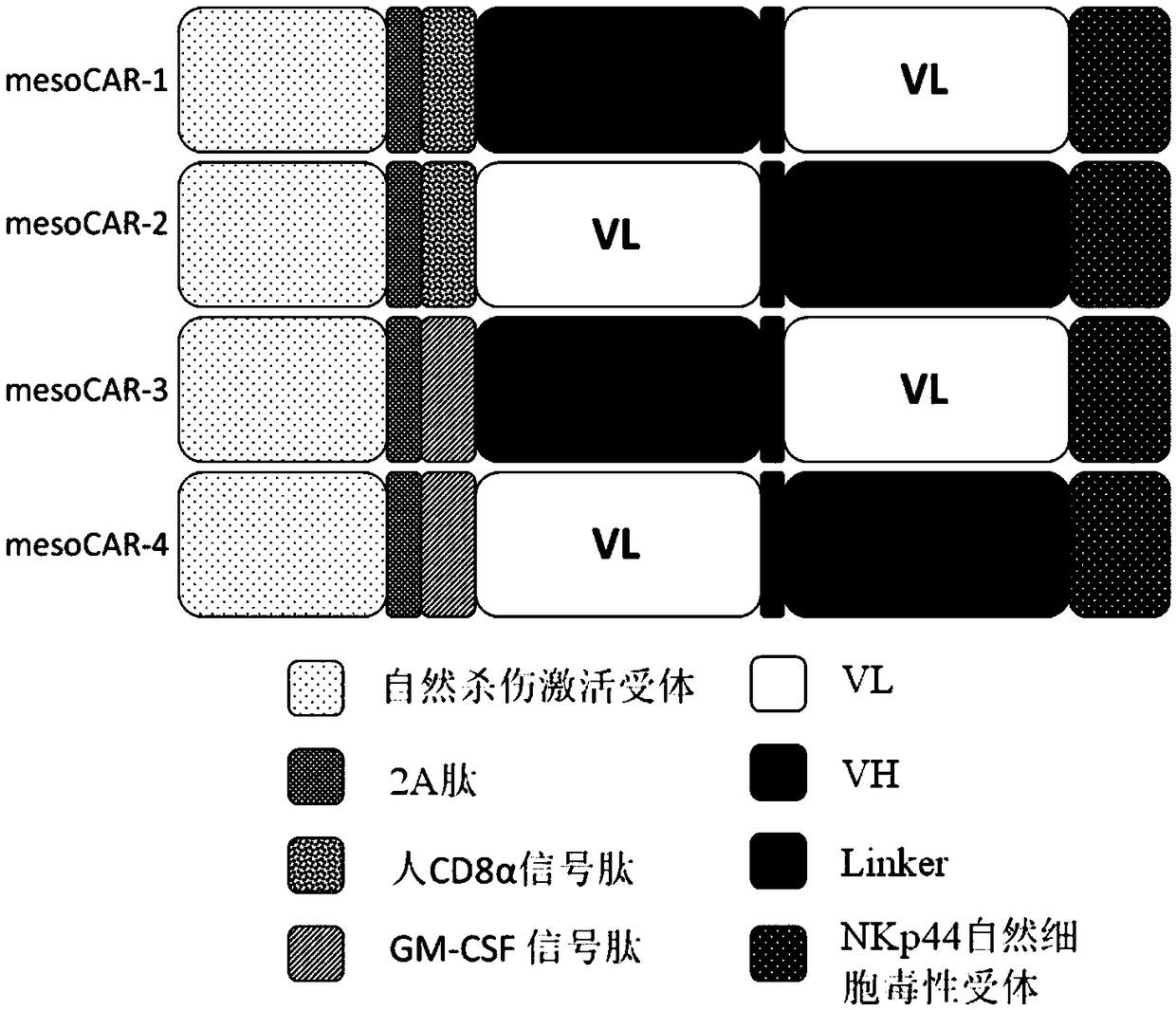
[0051] The present invention provides an optimized chimeric antigen receptor targeting MSLN antigen. The chimeric antigen receptor of the present invention is composed of the second intracellular conduction domain-T2A-extracellular signal peptide-targeting MSLN antigen binding domain-the first intracellular conduction domain in series. Therefore, viral vectors containing different combinations of stimulation signals need to be constructed separately. In this example, NKp44 is used as the unified structure of the first intracellular conduction domain, and the following four chimeric antigen receptors need to be constructed respectively ( figure 1 ):
[0052] DAP12-T2A-CD8α signal peptide-VH-Linker-VL-NKp44 (mesoCAR-1)
[0053] DAP12-T2A-CD8α signal peptide-VL-Linker-VH-NKp44 (mesoCAR-2)
[0054] DAP12-T2A-GM-CSF signal peptide-VH-Linker-VL-NKp44 (mesoCAR-3)
[0055] DAP12-T2A-GM-CSF signal peptide-VL-Linker-VH-NK...
Embodiment 2
[0101] Embodiment 2, virus infection T cell
[0102] 1. Isolation and activation of T cells and virus infection
[0103] (1) Isolation of human peripheral blood mononuclear cells
[0104] Use a blood collection tube containing anticoagulant to collect about 10ml of peripheral blood, settle naturally at room temperature (18-25°C) for about 30min, collect the upper layer of plasma, and centrifuge the collected upper layer of plasma at 5000r / min for 10min at a volume ratio of 1:1 Add to the lymphocyte separation medium (purchased from Tianjin Haoyang Biological Products Technology Co., Ltd.), gradient centrifugation, 3000r / min, centrifugation for 30min, after centrifugation, the centrifuge tube is layered from top to bottom: the first layer is the plasma layer ; The second layer is the buffy coat layer of lymphocytes; the third layer is the transparent separation liquid layer; the fourth layer is the red blood cell layer. Aspirate the buffy coat of lymphocytes, wash twice with ...
Embodiment 3
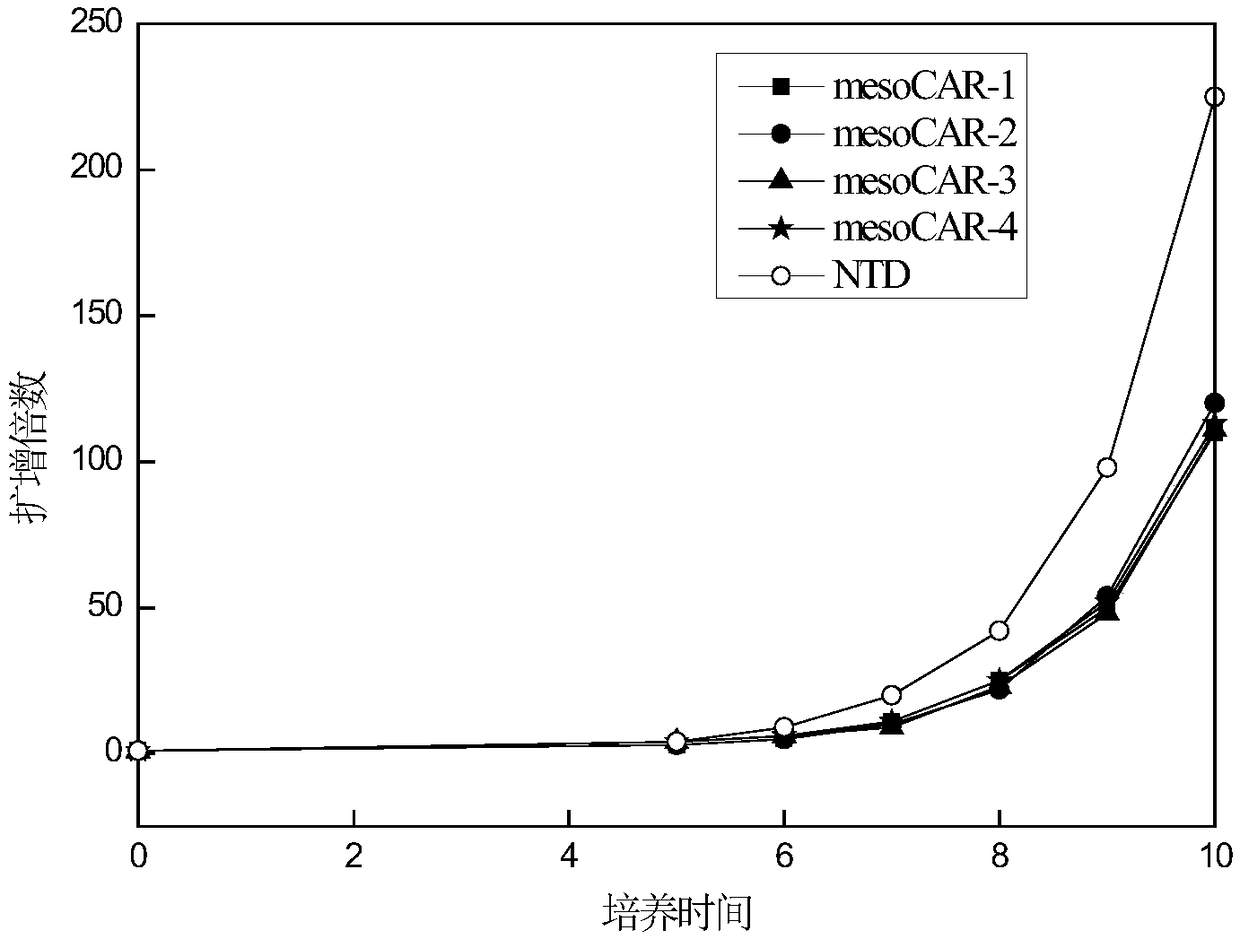
[0109] Example 3. Effect of Virus Infection of CAR-T Cells on Cell Proliferation
[0110] After each group of viruses infected T cells, the T cells were counted every 1-2 days with a complete medium containing 5% autologous plasma + 300 IU / ml recombinant human IL-2 + KBM581. Then observe the growth of T lymphocytes, the results are as follows: image 3 shown. The results showed that after the cells were infected with CAR-expressing viruses, they could still form typical proliferative clonal clusters. By counting the cells and drawing the cell proliferation curves, it could be seen that mesoCAR-1, mesoCAR-2, mesoCAR-3, and mesoCAR-4 proliferated similarly, compared with T cells uninfected with the virus ( image 3 Medium NTD) proliferative ability was slightly weaker.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Titer | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com