A kind of erbb2 single-chain antibody, chimeric antigen receptor targeting human erbb2, recombinant vector, recombinant cell and application
A technology of chimeric antigen receptors and single-chain antibodies, which is applied in the field of tumor immunobiological therapy, can solve the problems of unsustainable killing of tumor cells and large toxic and side effects, and achieve the ability of lasting multiple times of killing tumor cells and the release of cytokines Mild reaction, good continuous killing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
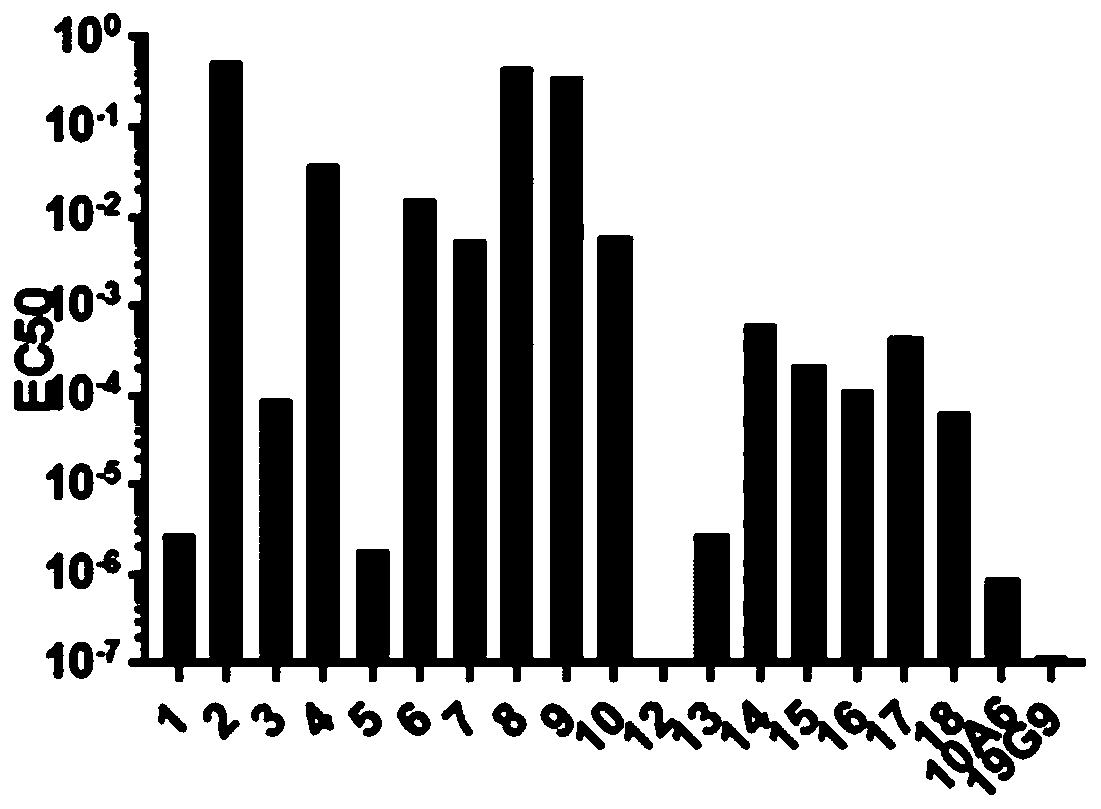
Embodiment 1
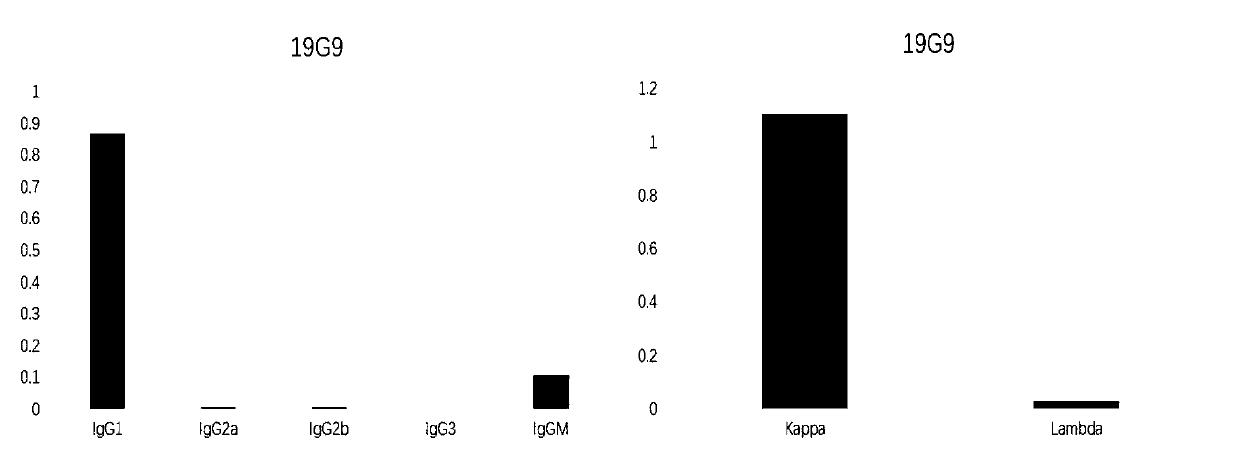
[0044] Preparation of ErbB2 Monoclonal Antibody 19G9
[0045] S1, synthetic ErbB2-Domain4 gene
[0046] The nucleotide sequence of the ErbB2-Domain4 gene is as follows:
[0047] atggagctggcggccttgtgccgctgggggctcctcctcgccctcttgccccccggagccgcgagcacctgccaccagctgtgcgcccgagggcactgctggggtccagggcccacccagtgtgtcaactgcagccagttccttcggggccaggagtgcgtggaggaatgccgagtactgcaggggctccccagggagtatgtgaatgccaggcactgtttgccgtgccaccctgagtgtcagccccagaatggctcagtgacctgttttggaccggaggctgaccagtgtgtggcctgtgcccactataaggaccctcccttctgcgtggcccgctgccccagcggtgtgaaacctgacctctcctacatgcccatctggaagtttccagatgaggagggcgcatgccagccttgccccatcaactgcacccactcctgtgtggacctggatgacaagggctgccccgccgagcagagagccagccctctgacgtccatcatctctgcggtggttggcattctgctggtcgtggtcttgggggtggtctttgggatcctcatc(SEQ ID No.21)
[0048] During the synthesis process, restriction endonucleases NdeI and XhoI were respectively added to the upstream and downstream of the ErbB2-Domain4 gene to obtain the ErbB2-Domain4 gene insertion fragment.
[0049] Expression ...
Embodiment 2
[0081] Preparation of recombinant vector
[0082] Arranging the expression sequence as GM-CSF signal peptide-19G9 single chain antibody region-CD8 transmembrane region-CD28 intracellular region-4-1BB intracellular region-CD3ζ intracellular region (GM-CSF signal peptide-19G9 ScFv-CD8TM-41BBcyto -CD28cyto-CD3ζ) form. Each fragment sequence is:
[0083] GM-CSF signal peptide (SEQ ID No.16)
[0084] cttctcctggtgacaagccttctgctctgtgagttaccacacccagcattcctcctgatccca
[0085] 19G9 single chain antibody region (SEQ ID No.2)
[0086] ctggagcagtcaggccctgggatattgcagccctcccagaccctcagtttgatctgttctttctctgggttttcactgagtacttctggtatgggtgtaagctggattcgccagccttcaggaaagggtctggagtggctggcacacatttactgggatgatgacaagcgctataacccatccctgaagagccggctcacaatctccaaggatacctccagcaagcaggttttcctcaagatcaccagtgttgacactgcagatactgccacatactactgtgctctcttcgcctactataactacgacgggactgtggactactggggtctaggaacctcagtcaccgtctcctcaggcggaggcggatcaggtggtggcggatctggaggtggcggaagcgacattgtgatgacacagactccatcctccctggctgtgtcagtaggagagaaggtc...
Embodiment 3
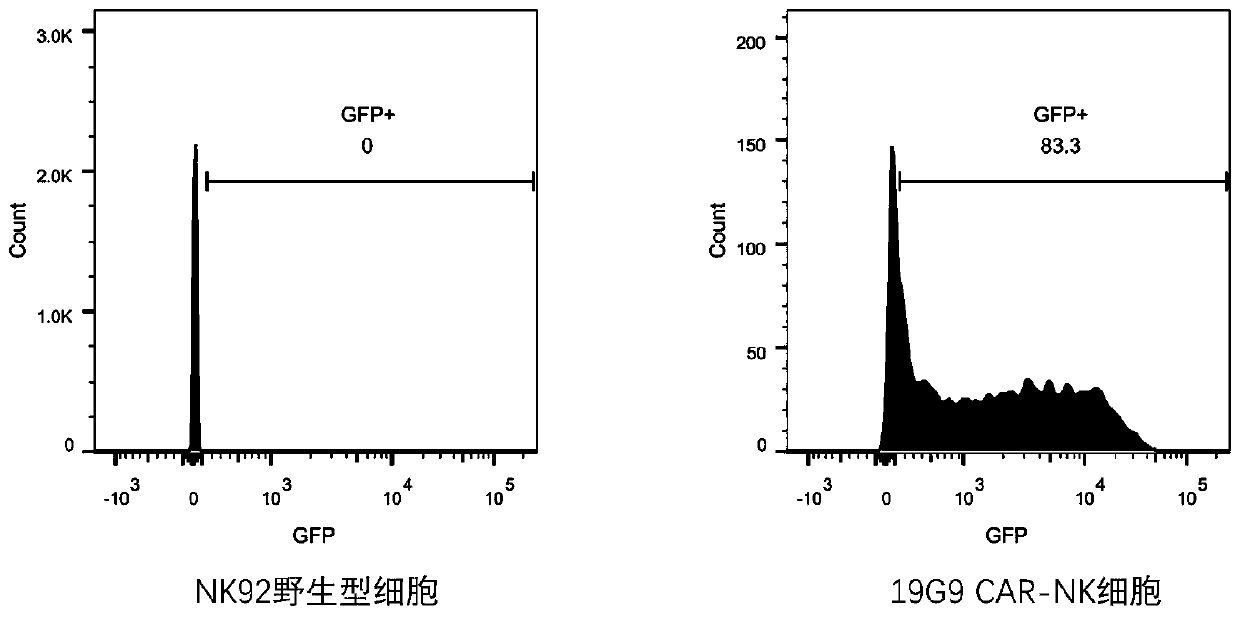
[0097] Preparation of recombinant cells
[0098] The packaged virus in Example 2 was used to infect NK92 cells (purchased from ATCC) at an MOI (multiplicity of infection) of 100:1. The infected NK92 cells were amplified and cultured in LY09 medium, and after 72 hours of culture, cells positive for the copGFP reporter gene (green fluorescence) were sorted by flow cytometry. At this time, the recombinant cell 19G9 CAR-NK was obtained. The results of flow cytometry analysis of GFP expression in 19G9 CAR-NK cells and wild-type NK92 cells after sorting were as follows: image 3 As shown, the expression level of GFP in 19G9 CAR-NK cells is high, while the expression level of GFP in wild-type NK92 cells is zero.
[0099] The specific binding of ErbB2 protein to 19G9 CAR-NK cells was analyzed by flow cytometry. Take 1 μg of ErbB2-biotin (Acro) protein and incubate with 1,000,000 19G9 CAR-NK cells and 1,000,000 NK92 cells for 30 minutes respectively, add Pacific Blue-labeled strepta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com