Bosentan tablet composition
A composition and technology for tablets, applied in the field of bosentan compositions, can solve the problems of loose tablets, drug quality risks, and related substances, etc. Effects of Dissolution Requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
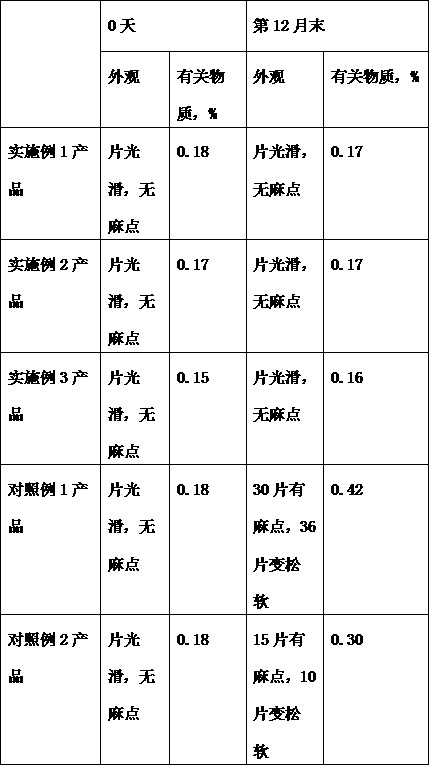
[0017] Example 1. Bosentan 62.5g, anhydrous lactose 78g, calcium hydrogen phosphate 8g, mannitol 10g, sodium lauryl sulfate 0.1g, micropowder silica gel 12g, microcrystalline cellulose 20g, croscarmellose Sodium 1.5g, magnesium stearate 0.8g. Prepare 1000 tablets according to the preparation method described in the technical scheme of this specification.
Embodiment 2
[0018] Example 2. Bosentan 62.5g, anhydrous lactose 48g, calcium hydrogen phosphate 16g, mannitol 20g, sodium lauryl sulfate 0.8g, micropowder silica gel 8g, microcrystalline cellulose 32g, croscarmellose Sodium 1.5-4g, magnesium stearate 0.8-1.6g. Prepare 1000 tablets according to the preparation method described in the technical scheme of this specification.
Embodiment 3
[0019] Example 3. Bosentan 62.5g, anhydrous lactose 67g, calcium hydrogen phosphate 12g, mannitol 15g, sodium lauryl sulfate 0.7g, micropowder silica gel 11g, microcrystalline cellulose 28g, croscarmellose Sodium 2.5g, magnesium stearate 1.3g. Prepare 1000 tablets according to the preparation method described in the technical scheme of this specification.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com