Application of bacteroides fragilis extract to preparation of medicines or foods for prevention and treatment of irritable bowel syndrome
A technology for irritable bowel syndrome and Bacteroides fragilis, applied in the field of medicine or food, can solve the problems that IBS cannot be completely cured, and there is a lack of treatment methods for IBS.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
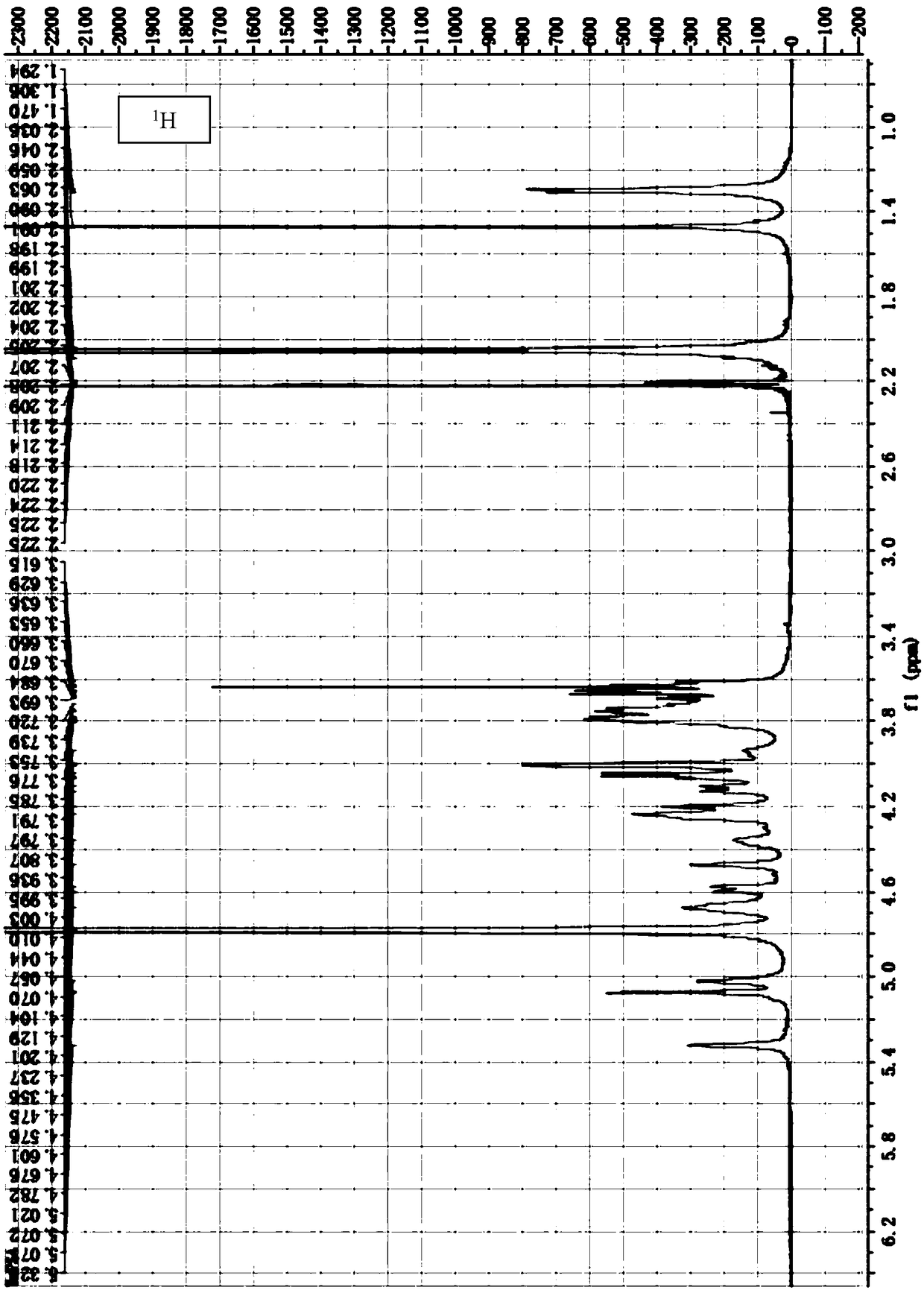
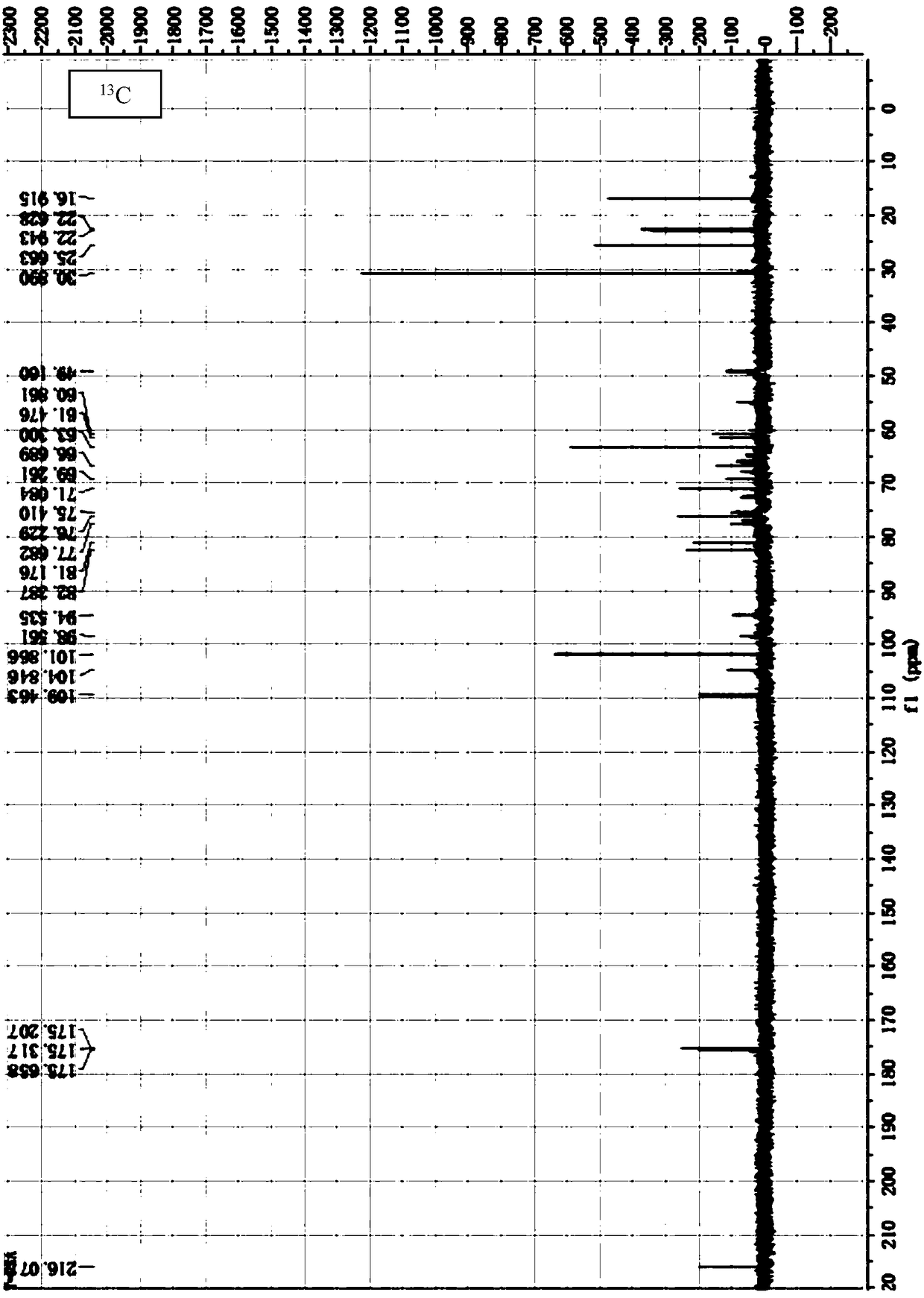
[0073] The preparation of embodiment 1 Bacteroides fragilis extract
[0074] (1) Fermentation culture of Bacteroides fragilis
[0075] Streak inoculation of strains on blood plate, anaerobic culture for 48h. Observe the colony morphological characteristics, staining characteristics, size, club shape and distribution, etc.
[0076] Colony characteristics: Bacteroides fragilis ZY-312, after being cultured on a blood plate for 48 hours, is slightly convex, translucent, white, smooth, non-hemolytic, and the diameter of the colony is between 1-3mm. See figure 1 .
[0077] Morphology under the microscope: Bacteroides fragilis ZY-312 was examined by Gram staining. It is a Gram-negative bacterium with a typical rod shape, blunt rounded ends and dense staining. The uncolored part in the middle of the bacteria is like a vacuole. figure 2 .
[0078] A single colony was selected and inoculated in tryptone broth for fermentation and culture for 8 hours (at a temperature of 37° C.). Th...
Embodiment 2
[0087] Example 2 Effect of Bacteroides fragilis capsular polysaccharide A on Senna-induced diarrhea-type IBS
[0088] 1. Experimental design
[0089] In order to verify the effect of the Bacteroides fragilis extract (the main component is capsular polysaccharide A) provided in Example 1 on the prevention / treatment of irritable bowel syndrome, 60 C57BL / 6 mice were selected for the experiment in this example. 60 C57BL / 6 mice were divided into male and female, and each experimental mouse was assigned a unique number. Prior to grouping animals, the item number, species / strain, sex, cage number, and animal number should be marked on the cage label. Use BioBook software to carry out random grouping according to the initial body weight of C57BL / 6 mouse, be divided into 6 groups, namely normal group (Group1), model group (Group2), loperamide capsule group (2.4mg / kg) (Group3), Bacteroides fragilis capsular polysaccharide A low (Group4), medium (Group5), high (Group6) dose groups, 10 ...
Embodiment 3
[0099] Example 3 Effect of Bacteroides fragilis capsular polysaccharide A on castor oil-induced diarrhea-type IBS
[0100] 1. Experimental design
[0101] In order to verify the effect of the Bacteroides fragilis extract (the main component is capsular polysaccharide A) provided in Example 1 on the prevention / treatment of irritable bowel syndrome, 60 C57BL / 6 mice were selected for the experiment in this example. 60 C57BL / 6 mice were divided into male and female, and each experimental mouse was assigned a unique number. Prior to grouping animals, the item number, species / strain, sex, cage number, and animal number should be marked on the cage label. Use BioBook software to carry out random grouping according to the initial body weight of C57BL / 6 mouse, be divided into 6 groups, namely normal group (Group1), model group (Group2), loperamide capsule group (2.4mg / kg) (Group3), Bacteroides fragilis capsular polysaccharide A low (Group4), medium (Group5), high (Group6) dose groups...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com