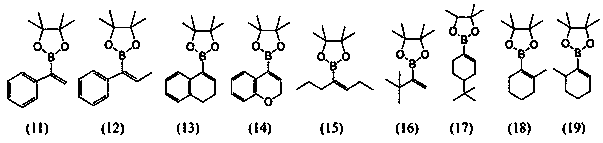
Preparation method of allylboronic acid pinacol ester
A technology of pinacol ester and alkenyl boronic acid, which is applied in the general preparation field of alkenyl boronic acid pinacol ester, and can solve problems such as potential safety hazards in the production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
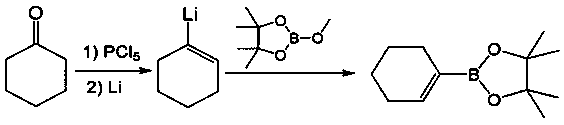
[0018] [Example 1] Preparation of cyclohexene-1-boronic acid pinacol ester
[0019]
[0020] After mixing cyclohexanone (20g, 0.2mol), pyridine p-toluenesulfonate (5g, 0.02mol), p-toluenesulfonyl hydrazide (38g, 0.2mol), and acetonitrile (200mL), the temperature was raised to reflux, and after the reaction , acetonitrile was distilled off under reduced pressure, 49 g of sulfonylhydrazone compound was obtained after adding isopropanol for recrystallization, and the yield was 90%;
[0021] Mix sulfonylhydrazone compound (49g, 0.18mol), isopropylmagnesium chloride solution (370mL, 1.0M) and tetramethylethylenediamine (23g, 0.2mol) and react at 40°C for 3h. After the reaction is complete, drop Add methylboronic acid pinacol ester (26.5g, 0.19mol), react for 2h, then heat up to reflux for 1h. After the reaction was completed, after the solvent was evaporated, the product cyclohexene-1-boronic acid pinacol ester was continuously evaporated at about 100° C. to obtain 27.5 g, with...
Embodiment 2
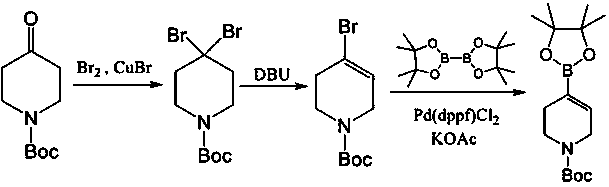
[0022] [Example 2] Preparation of 1-ethyl-1,2,3,6-tetrahydropyridine-4-boronic acid pinacol ester
[0023]
[0024] After mixing N-ethyl-4-piperidone (25g, 0.2mol), pyridine p-toluenesulfonate (5g, 0.02mol), p-toluenesulfonyl hydrazide (40g, 0.21mol) and ethanol (250mL), The temperature was raised to reflux, and after the reaction was completed, the solvent was evaporated, and 50 g of the sulfonyl hydrazone compound recrystallized from isopropanol was added, with a yield of 86%;
[0025] Mix the compound hydrazone (50g, 0.17mol), isopropylmagnesium chloride solution (430mL, 1.0M), and tetramethylethylenediamine (22g, 0.19mol) and react under reflux for 3h. After the reaction is complete, add methyl Boric acid pinacol ester; (24g, 0.17mol), react for 2h, then heat up to reflux for 1h. After the reaction was completed, after the solvent was evaporated, the product 1-ethyl-1,2,3,6-tetrahydropyridine-4-boronic acid pinacol ester was continuously evaporated to obtain 25.5 g, wi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com