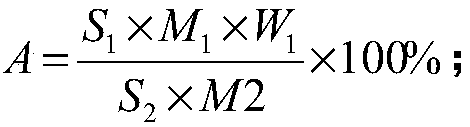Method for purifying aminopyrimidine
A technology of aminopyrimidine and purification method, which is applied in the field of aminopyrimidine purification, and can solve the problems of low yield and quality of vitamin B1
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] A method for purifying aminopyrimidine, the steps of which are:
[0041] a. Formylpyrimidine: sodium hydroxide: water = 1:2:4 ratio, heat up and dissolve, keep warm at 100°C for 3 hours to obtain aminopyrimidine solution;
[0042] b. Cool down to 5-10°C, the aminopyrimidine solid precipitates out, and the aminopyrimidine is filtered out;
[0043] c. Wash the aminopyrimidine solid with methanol to remove impurities, and the ratio of aminopyrimidine to methanol is 1:2;
[0044] d. Filter out aminopyrimidine, add water and heat up to 70°C to dissolve, the ratio of aminopyrimidine to water is 1:0.5;
[0045] e. The temperature is lowered to 5-10° C., the aminopyrimidine solid is precipitated, and the pure aminopyrimidine is obtained by filtering and drying.
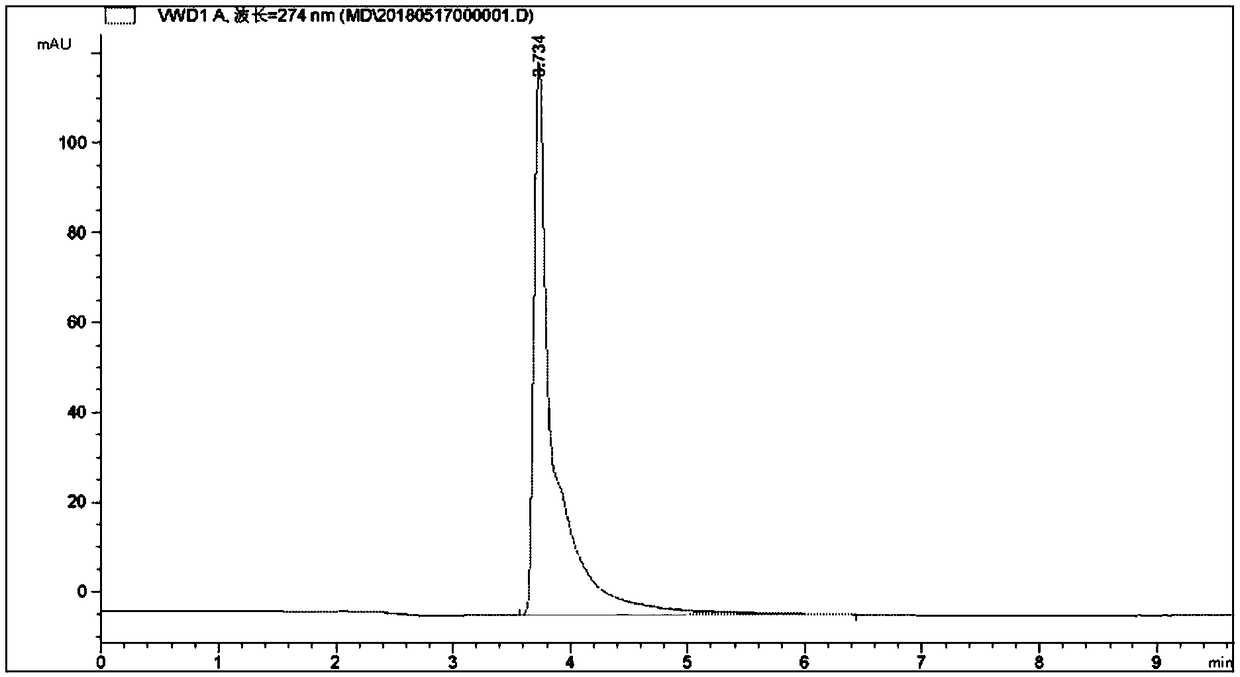
[0046] f, the aminopyrimidine pure product that obtains detects, as figure 1 shown.
[0047] figure 1 The information in it is shown in Table 1.
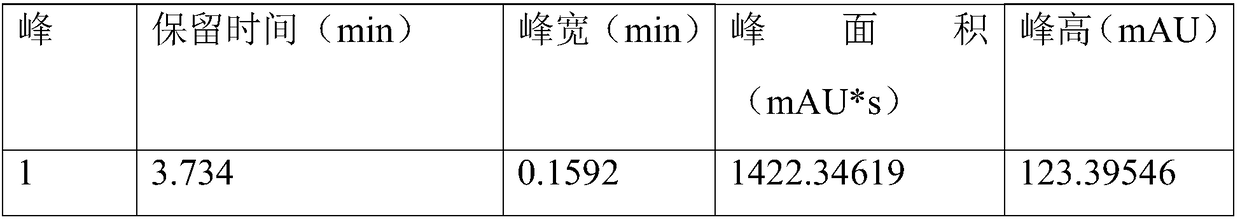
[0048] Table 1 Detection peak information
[0049]
[0050] A...
Embodiment 2
[0060] A method for purifying aminopyrimidine, the steps of which are:
[0061] a. Formylpyrimidine: sodium hydroxide: water = 1:1:3 ratio, heat up and dissolve, keep warm at 80°C for 4 hours to obtain aminopyrimidine solution;
[0062] b. Cool down to 0-5°C, the aminopyrimidine solid precipitates out, and the aminopyrimidine is filtered out;
[0063] c. Wash the aminopyrimidine solid with methanol to remove impurities, and the ratio of aminopyrimidine to methanol is 1:2;
[0064] d. Filter out aminopyrimidine, add water and heat up to 50°C to dissolve, the ratio of aminopyrimidine to water is 1:0.5;
[0065] e. The temperature is lowered to 0-5° C., the aminopyrimidine solid is precipitated, and the pure aminopyrimidine is obtained by filtering and drying.
[0066] f, the obtained pure aminopyrimidine is detected.
[0067] Aminopyrimidine content, i.e. aminopyrimidine purity, is calculated as follows:
[0068]
[0069] Wherein, A—aminopyrimidine content;
[0070] S 1...
Embodiment 3
[0077] A method for purifying aminopyrimidine, the steps of which are:
[0078] a. Formylpyrimidine: sodium hydroxide: water = 1:2:5 ratio, heat up and dissolve, keep warm at 120°C for 2 hours to obtain aminopyrimidine solution;
[0079] b. Cool down to 10-20°C, the aminopyrimidine solid precipitates out, and the aminopyrimidine is filtered out;
[0080] c. Wash the aminopyrimidine solid with methanol to remove impurities, and the ratio of aminopyrimidine to methanol is 1:2;
[0081] d. Filter out aminopyrimidine, add water and heat up to 80°C to dissolve, the ratio of aminopyrimidine to water is 1:0.5;
[0082] e. The temperature is lowered to 10-20° C., the aminopyrimidine solid is precipitated, and the pure aminopyrimidine is obtained by filtering and drying.
[0083] f, the obtained pure aminopyrimidine is detected.
[0084] Aminopyrimidine content, i.e. aminopyrimidine purity, is calculated as follows:
[0085]
[0086] Wherein, A—aminopyrimidine content;
[0087]...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com