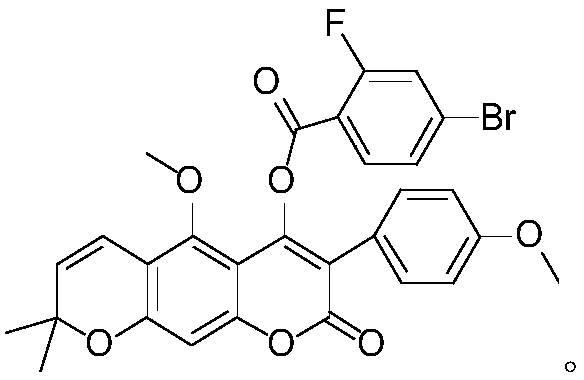
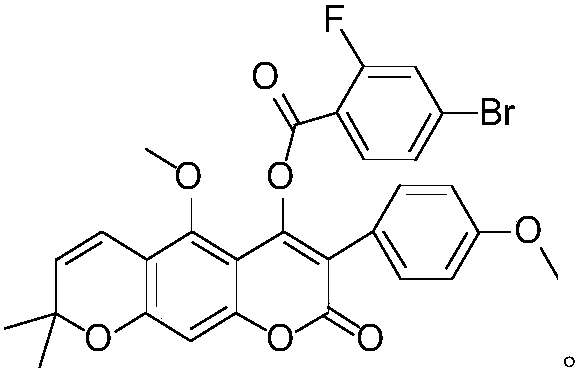
O-(4-bromo-2-fluorobenzoyl) 3-phenylcoumarin robustic acid with anti-tumor activity and preparation and application methods thereof
A technology of fluorobenzoyl and anti-tumor activity, which is applied in the field of medicine, can solve the problems of no tumor inhibitory effect and cross-drug resistance, and achieve the effect of simple preparation method and simple purification method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
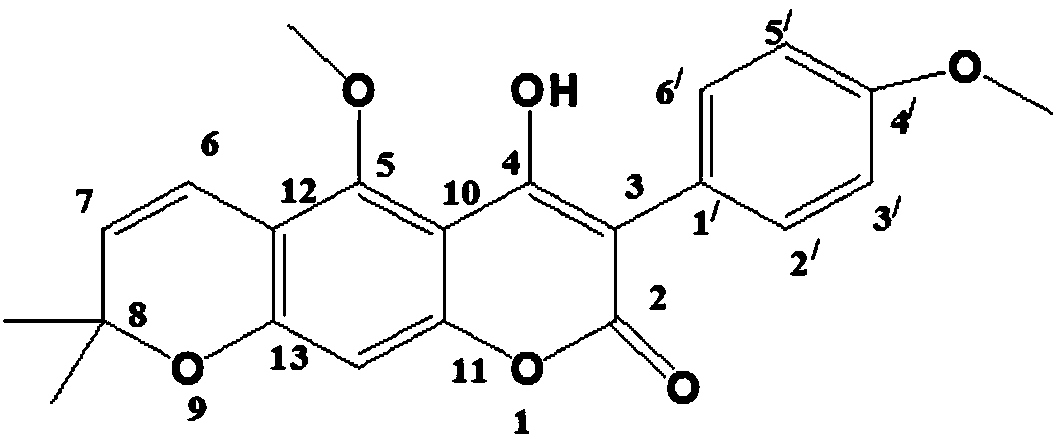
[0025] O-(4-bromo-2-fluorobenzoyl) licorice A with anti-tumor activity, its chemical structural formula is as follows:
[0026]
[0027] The preparation method of O-(4-bromo-2-fluorobenzoyl) licorice A with antitumor activity comprises the following steps:
[0028] Add 5 times the mass of acetonitrile to 190 mg of licorice A (0.5 mmoL) to dissolve, add pyridine and 2-fluoro-4-bromobenzoyl chloride, control the reaction temperature between 60-80 ° C and heat under reflux for 35 minutes to obtain The reaction product, the reaction product is evaporated to remove the solvent under reduced pressure to obtain a residue, and the residue is recrystallized to obtain O-(4-bromo-2-fluorobenzoyl) licorice A, wherein, licorice A, pyridine, 2-fluoro The molar ratio of -4-bromobenzoyl chloride is 1:0.1:2, and the solvent used for recrystallization of the residue is absolute ethanol.
[0029] O-(4-bromo-2-fluorobenzoyl)glycyrrhizae A is a white powdery solid with a yield of 61.03%.
[0...
Embodiment 2
[0032] O-(4-bromo-2-fluorobenzoyl) licorice A with anti-tumor activity, its chemical structural formula is as follows:
[0033]
[0034] The preparation method of O-(4-bromo-2-fluorobenzoyl) licorice A with antitumor activity comprises the following steps:
[0035] Add 10 times the mass of acetonitrile to Glycyrrhizae A to dissolve, add pyridine and 2-fluoro-4-bromobenzoyl chloride, control the reaction temperature between 60-80°C and heat under reflux for 30 minutes to obtain the reaction product, the reaction product The solvent was distilled off under reduced pressure to obtain a residue, and the residue was recrystallized to obtain O-(4-bromo-2-fluorobenzoyl)glycyrrhizae A, wherein, licorice A, pyridine, 2-fluoro-4-bromobenzene The molar ratio of formyl chloride was 1:0.1:6, and the solvent used for recrystallization of the residue was acetonitrile.
[0036] O-(4-bromo-2-fluorobenzoyl)glycyrrhizae A is a white powdery solid with a yield of 63.43%.
Embodiment 3
[0038] O-(4-bromo-2-fluorobenzoyl) licorice A with anti-tumor activity, its chemical structural formula is as follows:
[0039]
[0040] The preparation method of O-(4-bromo-2-fluorobenzoyl) licorice A with antitumor activity comprises the following steps:
[0041] Add 15 times the mass of acetonitrile to Glycyrrhizae A to dissolve, add pyridine and 2-fluoro-4-bromobenzoyl chloride, control the reaction temperature between 60-80°C and heat under reflux for 35 minutes to obtain the reaction product, the reaction product The solvent was distilled off under reduced pressure to obtain a residue, and the residue was recrystallized to obtain O-(4-bromo-2-fluorobenzoyl)glycyrrhizae A, wherein Glycyrrhizae A, pyridine, 2-fluoro-4-bromobenzene The molar ratio of formyl chloride was 1:0.1:10, and the solvent used for recrystallization of the residue was ethyl acetate.
[0042] O-(4-bromo-2-fluorobenzoyl)glycyrrhizae A is a white powdery solid with a yield of 66.08%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com