Antibody capable of bonding with LINC0266-1
A technology of antibodies and peptides, applied in the field of cancer diagnosis and treatment, can solve the problems of non-existence of antibodies, and achieve the effects of increasing the positive rate, high mortality rate, and short survival period
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Prepare the anti-LINC00266-1 polypeptide antibody, and detect the titer of the prepared anti-LINC00266-1 polypeptide antibody, the specific operation process is as follows:
[0058] (1) The designed antigenic epitope peptide 1 (shown in SEQ ID NO: 2) and antigenic epitope peptide 2 (shown in SEQ ID NO: 3) against the LINC00266-1 polypeptide were synthesized by chemical synthesis (Gill Biochemical (Shanghai) Co., Ltd.), then the synthetic epitope peptide 1 and antigen epitope peptide 2 were coupled with keyhole limpet hemocyanin as immune antigen 1 and immune antigen 2, and were analyzed by high pressure liquid chromatography (HPLC). The immune antigen is separated and purified to make its purity >85%.
[0059] Take 2 healthy New Zealand rabbits with body weight not less than 2kg, named as the rabbit of antigenic epitope peptide 1 and the rabbit of antigenic epitope peptide 2 respectively. 2 to 3 ml of blood from the above-mentioned rabbit ear arteries were collected, a...
Embodiment 2
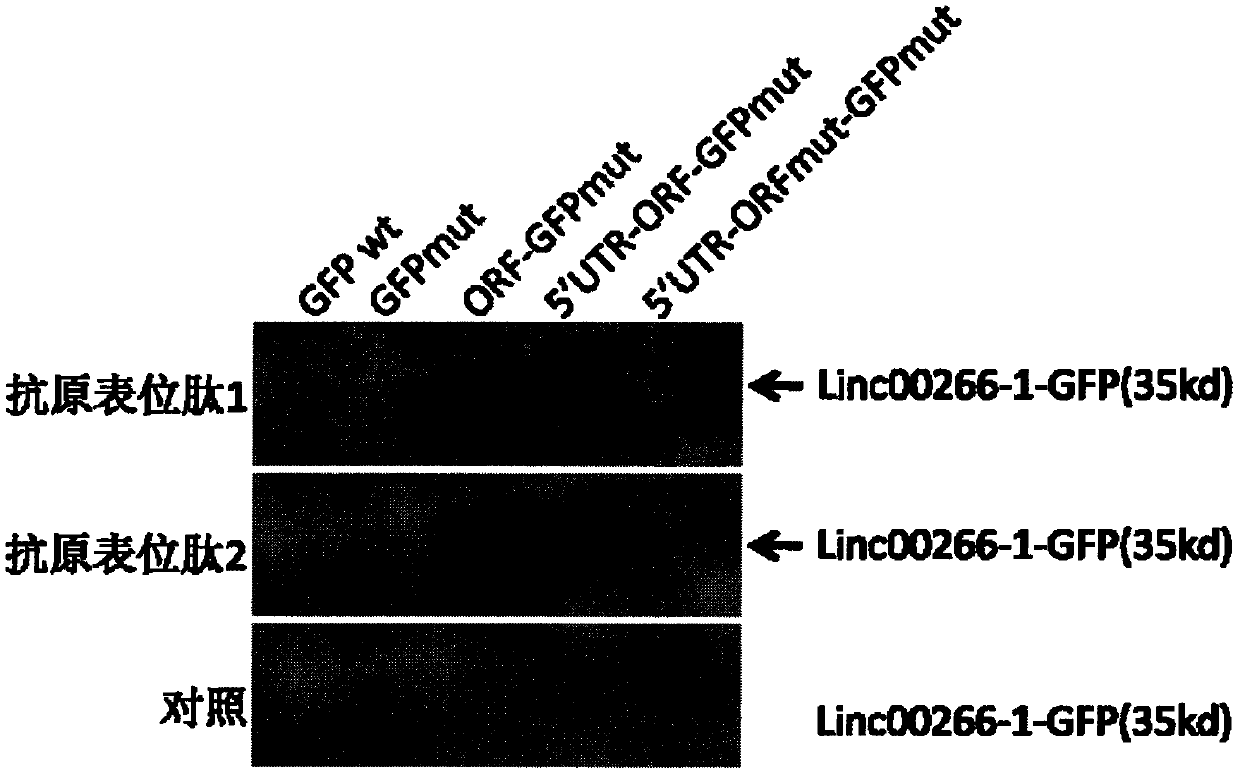
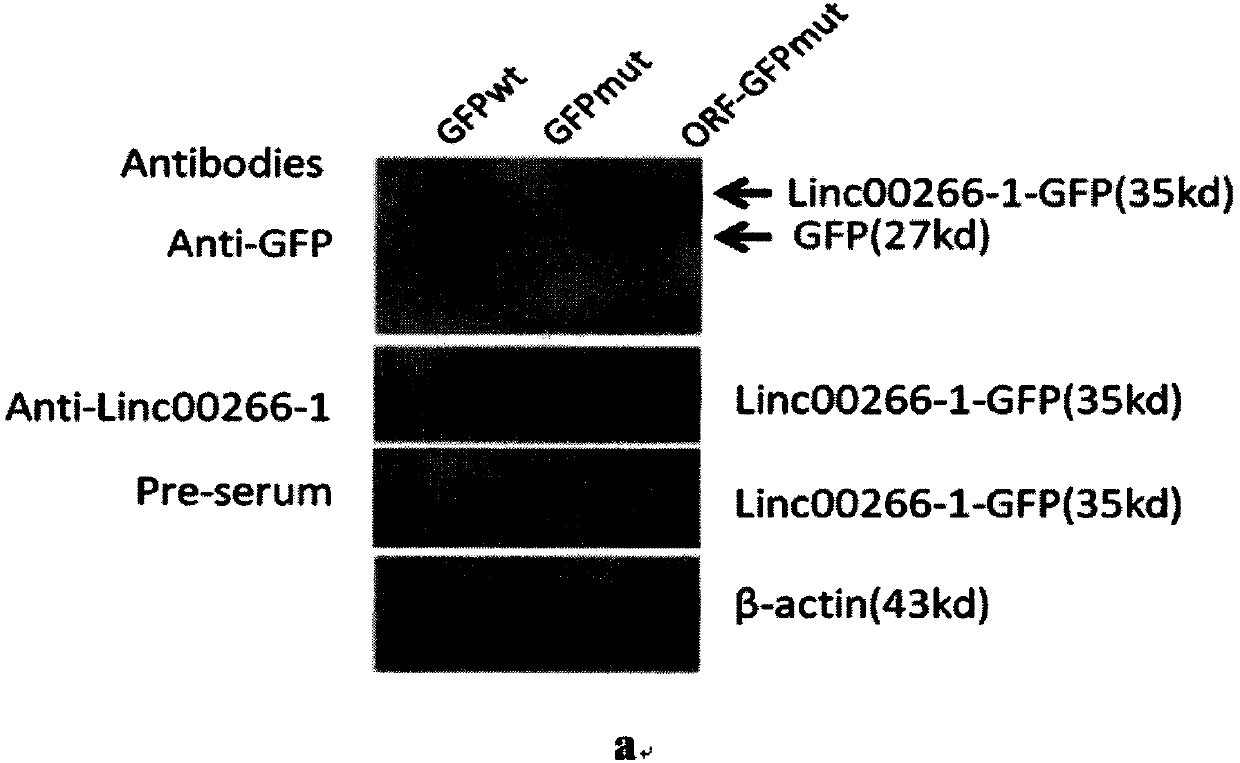
[0074] Anti-LINC00266-1 antibodies prepared based on epitope peptide 1 and anti-LINC00266-1 antibodies prepared based on epitope peptide 2 were used for specific detection experiments on LINC00266-1 polypeptides. The specific steps are as follows:
[0075] The first step is to construct the LINC00266-1-GFP fusion gene expression vector. First, site-directed mutation of the wild-type GFP (GFPwt) gene translation promoter ATGGTG sequence to ATTGTT (GFPmut). Therefore, the translation of the GFP gene is destroyed, and the translation promoter ATG on the LINC00266-1 gene is used for translation, thereby expressing and producing the LINC00266-1-GFP fusion protein. After transfecting HeLa cells (ATCC, Number: CCL-2) with liposome Lipo2000 (Life technology) for 24 hours, the cells were collected and lysed to prepare protein samples. Using the anti-LINC00266-1 polypeptide antibody prepared based on antigenic epitope peptide 1 and the anti-LINC00266-1 polypeptide antibody prepared bas...
Embodiment 3
[0080] Using the Western blotting method, the anti-LINC00266-1 antibody prepared by the present invention was used to detect the expression level of the LINC00266-1 polypeptide in colon cancer cells with different invasion and metastasis potentials from the same source. The specific process is as follows:
[0081] Two pairs of colon cancer cells SW480 and SW620 (ATCC, Number: CCL-228 and CCL-227, respectively), HTC-116 (ATCC, Number: CCL-247) and HTC-116high (this cell Established by the previous screening in our laboratory), the cells were lysed to prepare protein samples. Using the Western blotting method, the anti-LINC00266-1 polypeptide antibody prepared by the present invention was used to detect the difference in the level of LINC00266-1 polypeptide between the paired cells. The result is as image 3 As shown, the level of LINC00266-1 polypeptide was significantly up-regulated in colon cancer cells SW620 and HTC-116high with high invasion and metastasis potential. In t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com