Aureomycin microcapsule particles used for oral administration of ruminants and preparation method thereof
A ruminant, chlortetracycline technology, applied in microcapsules, medical preparations with inactive ingredients, capsule delivery, etc., can solve problems such as digestive disorders, animals do not ruminate, and achieve fewer processes, low production energy consumption, and flow rate. Good dispersion effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Embodiment 1 prepares aureomycin microcapsule preparation
[0048] The preparation steps are as follows:
[0049] S1. Weigh each auxiliary material according to the following formula ratio (based on the total mass as 100g):
[0050] Chlortetracycline premix 44%;
[0051] Hydrogenated oil 56%;
[0052] Heat to melt at 90°C and mix well;
[0053] S2. Add the aureomycin premix to the auxiliary material obtained in S1, and stir evenly to obtain a mixed material;
[0054] S3. Cool the mixed material obtained in S2 to 70-85°C, and carry out the stage of fluidized bed spray granulation and spheroidization. The temperature of the fluidized air in the granulation and spheroidization stage is 5°C. The linear velocity is 40 m / s;
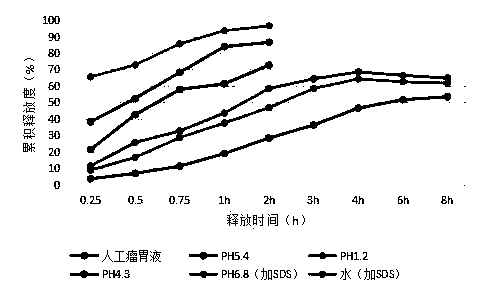
[0055] S4. Cool, sieve, and collect to obtain 10% aureomycin microcapsules, which are black spherical particles with a particle size of 250-850 μm.
Embodiment 2
[0056] Embodiment 2 prepares aureomycin microcapsule preparation
[0057] S1. Weigh each auxiliary material according to the following formula ratio (based on the total mass as 100g):
[0058] Chlortetracycline premix 44%;
[0059] Glyceryl monostearate 56%;
[0060] Heat to melt at 90°C and mix well;
[0061] S2. Add the aureomycin premix to the auxiliary material obtained in S1, and stir evenly to obtain a mixed material;
[0062] S3. Cool the mixed material obtained in S2 to 70-85°C, and carry out the stage of fluidized bed spray granulation and spheroidization. The temperature of the fluidized air in the granulation and spheroidization stage is 5°C. The linear velocity is 40 m / s;
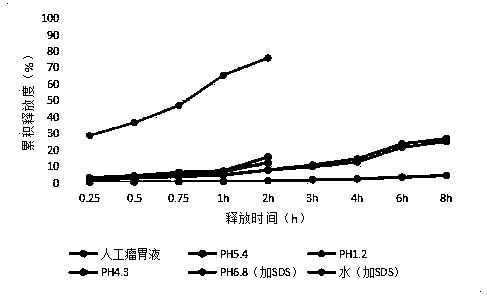
[0063] S4. Cool, sieve, and collect to obtain 10% aureomycin microcapsules, which are black spherical particles with a particle size of 250-850 μm.
Embodiment 3
[0064] Embodiment 3 prepares aureomycin microcapsule preparation
[0065] S1. Weigh each auxiliary material according to the following formula ratio (based on the total mass as 100g):
[0066] Chlortetracycline premix 44%;
[0067] PEG400056%;
[0068] Heat to melt at 90°C and mix well;
[0069] S2. Add the aureomycin premix to the auxiliary material obtained in S1, and stir evenly to obtain a mixed material;
[0070] S3. Cool the mixed material obtained in S2 to 70-85°C, and carry out the stage of fluidized bed spray granulation and spheroidization. The temperature of the fluidized air in the granulation and spheroidization stage is 5°C. The linear velocity is 45 m / s;
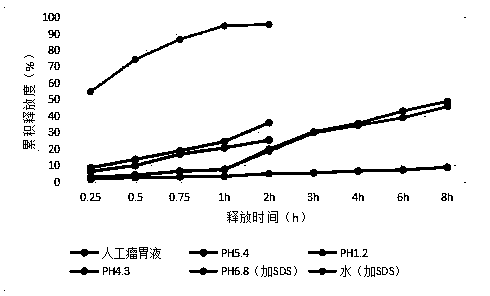
[0071] S4. Cool, sieve, and collect to obtain 10% aureomycin microcapsules, which are black spherical particles with a particle size of 250-850 μm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com