Method for detecting embryo health by using blastocyst culture solution, and product
A technology for health status and culture medium, which is applied in the fields of biomedicine and molecular cell biology, and can solve the problems of reduced proportion of embryo-derived DNA, inability to target at the same time, and inability to target IVF samples.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
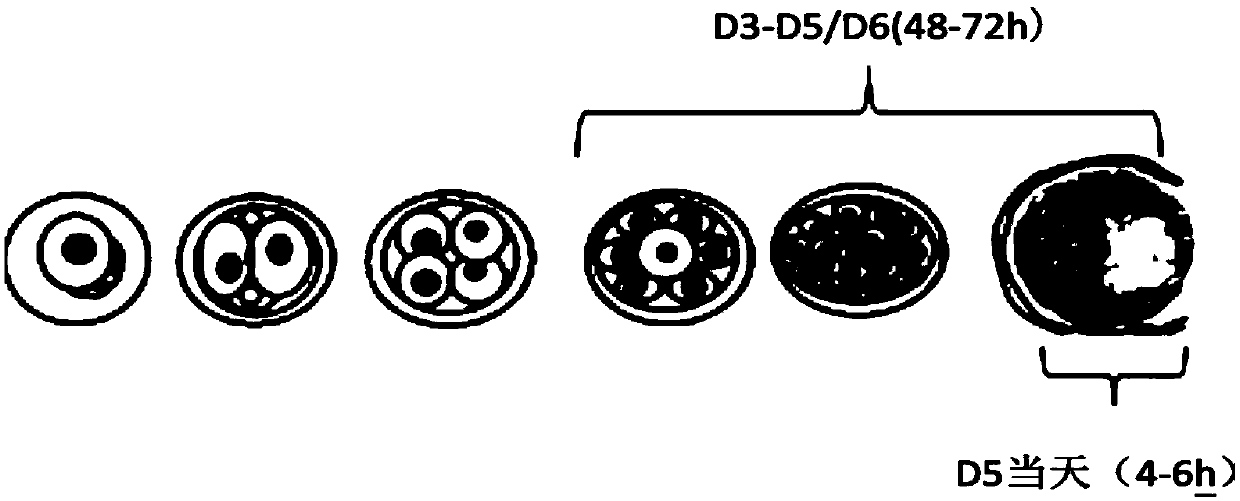
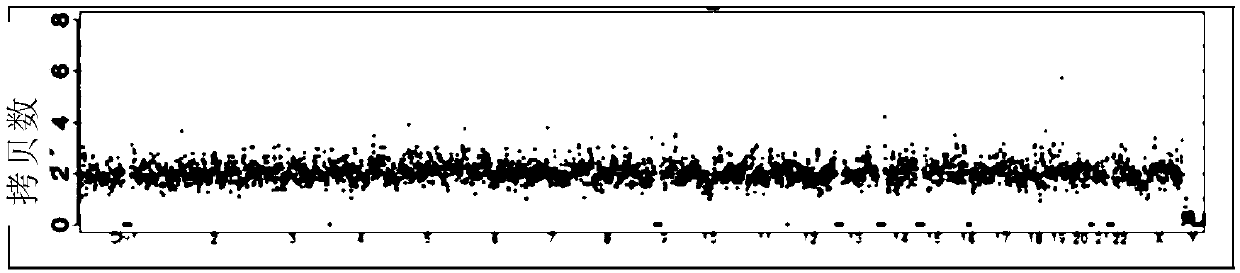
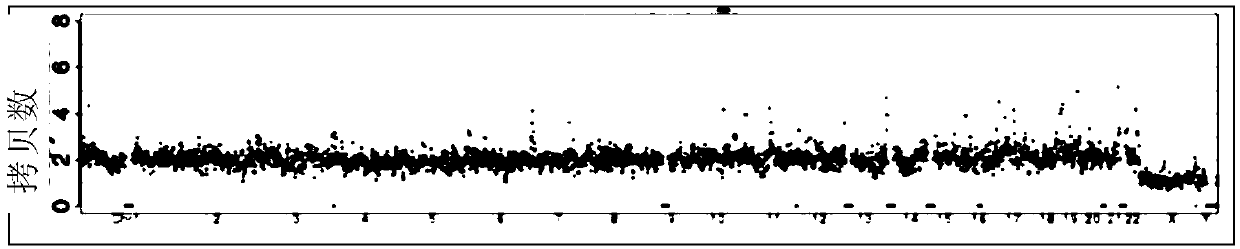
[0190] A large number of experimental data prove that the accuracy of the detection results of the present invention is significantly improved, and the false negative rate is reduced to below 5%. Compared with conventional D3-D5 sampling (130 groups of samples), and the comparison with PGS (62 groups of samples), the false negative rate as follows:
[0191]
D3-D5 sampling
D5 Sampling on the day
false negative rate
12.3%
1.6%
Accuracy
73.1%
91%
[0192] In D5, the new blastocyst culture medium is replaced by short-term culture and sampling, and the false negative rate can be controlled by 1.6%. However, the collection method of D3-D5 cannot effectively control the maternal interference, and the false negative rate is 12.3%, which is easy to cause clinical problems. misdiagnosis.
[0193] Among them, the false negative cases caused by D3-D5 are as follows:
[0194]
[0195] D5 of the present invention is replaced with new blas...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com