Microalbuminuria immunogen, preparation method of microalbuminuria immunogen, polyclonal antibody and preparation method and application of polyclonal antibody
A urine microalbumin and polyclonal antibody technology, applied in the field of biochemistry, can solve the problems of excess, missed detection, insufficient sensitivity and specificity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0023] The preparation method of urinary microalbumin immunogen of one embodiment, comprises the following steps:
[0024] S110, purifying urinary microalbumin in urine.
[0025] Specifically, the step of purifying urinary microalbumin in urine includes the following operations:
[0026] S111, rough and pure.
[0027] Specifically, urine is subjected to salting out to obtain a crude product of urinary microalbumin.
[0028] In one of the embodiments, the urine is subjected to ammonium sulfate salting-out treatment to obtain crude urine microalbumin. Further, the saturation of ammonium sulfate is 40%-55%. Furthermore, the saturation of ammonium sulfate is 40%, 50% or 55%. Of course, in some other embodiments, other salts (such as sodium chloride, sodium sulfate, etc.) can also be used to perform salting-out treatment on the urine. It can be understood that, the method of obtaining the crude urinary microalbumin product can also be other methods well known in the art except...
Embodiment 1
[0066] urinary microalbumin in purified urine
[0067] (1) The urine from clinical patients in the department of nephrology was subjected to 50% saturated ammonium sulfate salting-out treatment to precipitate some miscellaneous proteins, and the supernatant was collected to obtain crude urine microalbumin.
[0068] (2) Dialyze the crude product of urinary microalbumin into 10mM phosphate buffered saline, centrifuge, take the supernatant and put it on an affic-blue affinity chromatography column (filler affic-blue comes from Bio-Rad, an American company), and pass through 0 %~20% B (phosphate buffered saline containing 1.4M NaCl, PBS) for linear elution, 100% B for one-step elution, collect the eluted fractions of affic-blue100% affinity chromatography, and then pass through a ProteinG column to collect the flow Wear the sample to obtain intermediate purified urine microalbumin.
[0069] (3) The intermediate purified urine microalbumin was subjected to ultrafiltration and conc...
Embodiment 2
[0072] Preparation of urinary microalbumin immunogen
[0073] (1) Adjust the concentration of urinary microalbumin prepared in Example 1 to 3 mg / mL, and then treat it in a hot water bath at 55°C for 30 minutes, then immediately treat it in an ice bath at 0°C under dark conditions for 2 hours to obtain urinary microalbumin immunogen.
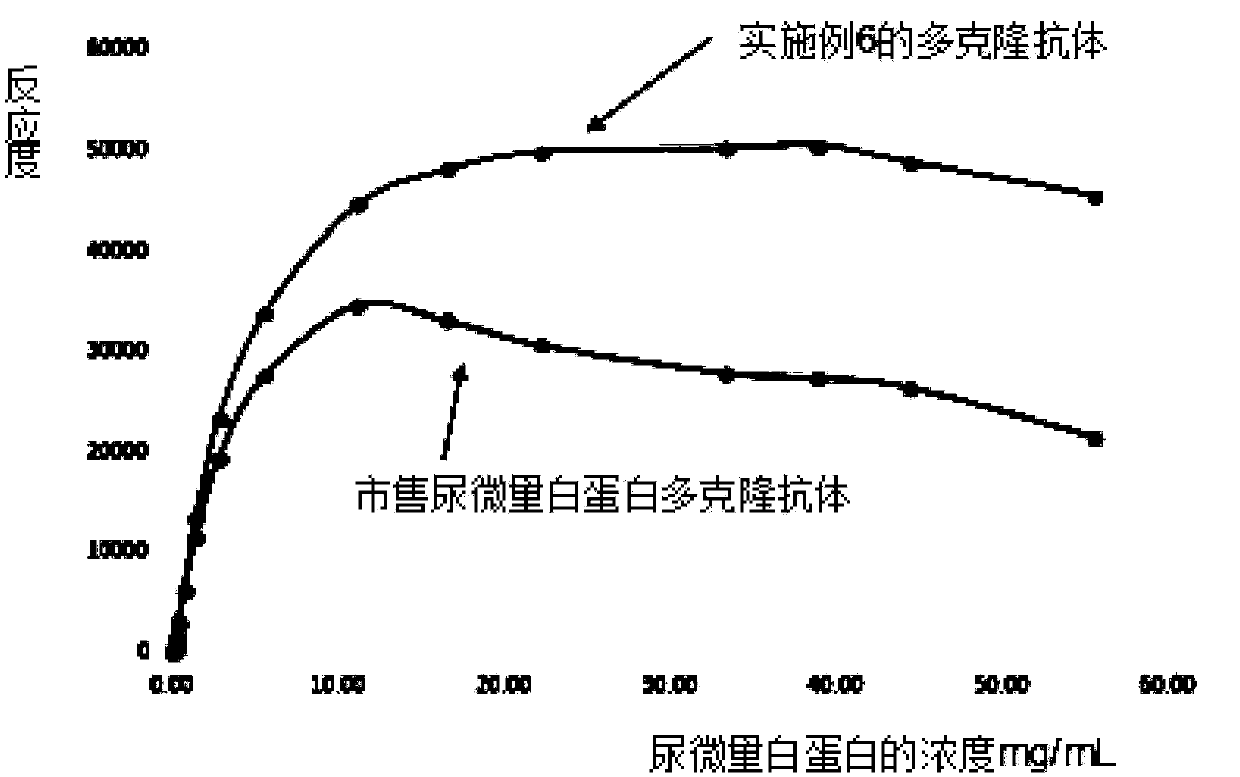
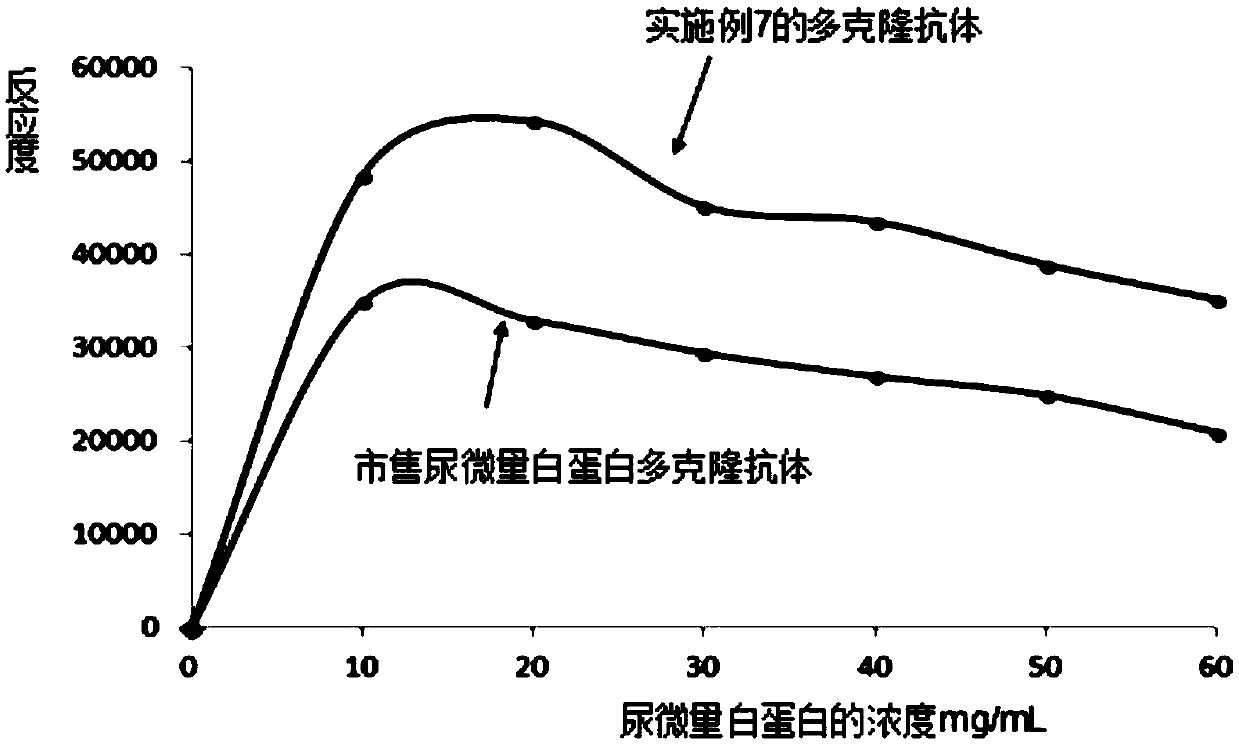
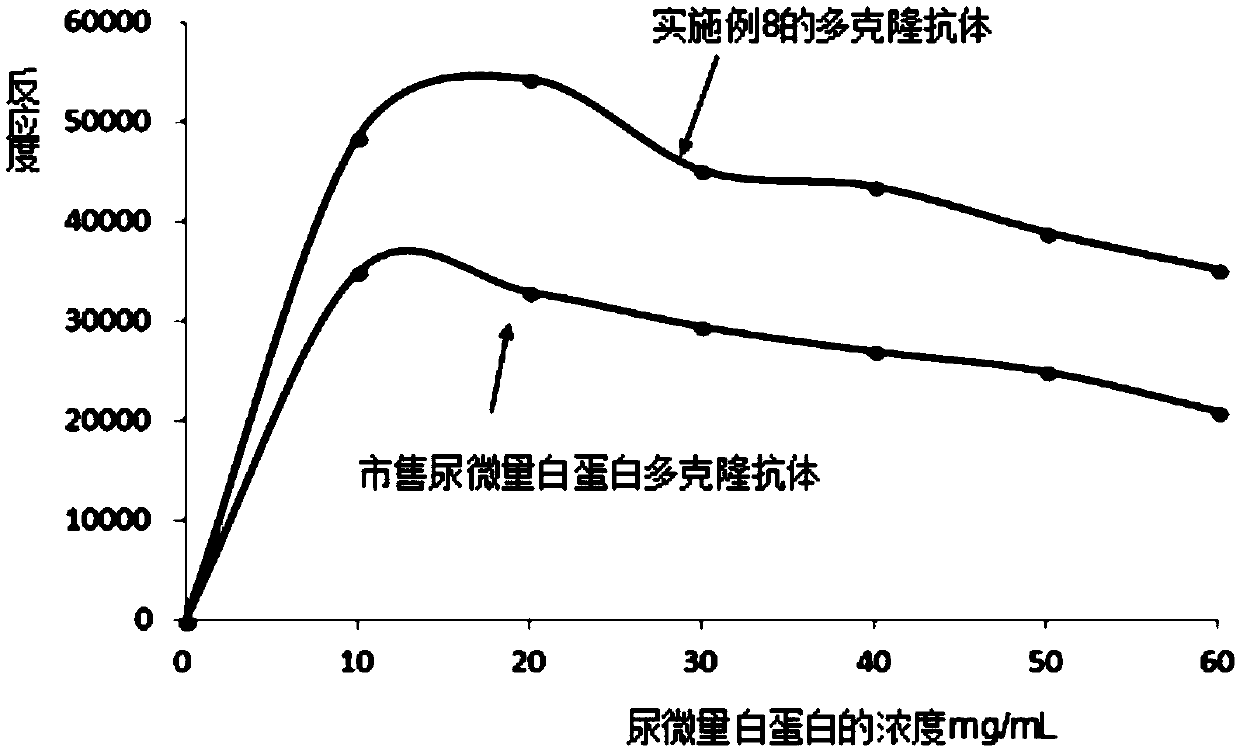
[0074] (2) Test the reactivity of the urinary microalbumin immunogen obtained in (1) by turbidimetry. The test results of Example 2 are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com