Method for selectively catalyzing hydrogenolysis of aryl group C-Br bond using nano porous metal
A nanoporous, metal catalyst technology, used in pharmaceutical chemical intermediates and related chemical fields, to achieve the effect of structural stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Embodiment 1, the preparation of 2-phenyl-1,3-dioxolane
[0023] Nanoporous metal gold catalyst (4.9mg, 0.025mmol), methanol (2mL) and 2-(2-bromophenyl)-1,3-dioxolane (57.26mg, 0.25mmol), cesium carbonate (364.3mg , 1.125mmol) was added to the reaction kettle, hydrogen gas (20bar) was introduced, heated and stirred, the reaction temperature was controlled at 90°C, the reaction time was controlled at 50h, and the reaction solution was extracted with water to obtain 2-phenyl-1,3-diox Pentacycline 33.8 mg, yield 90%.
[0024]
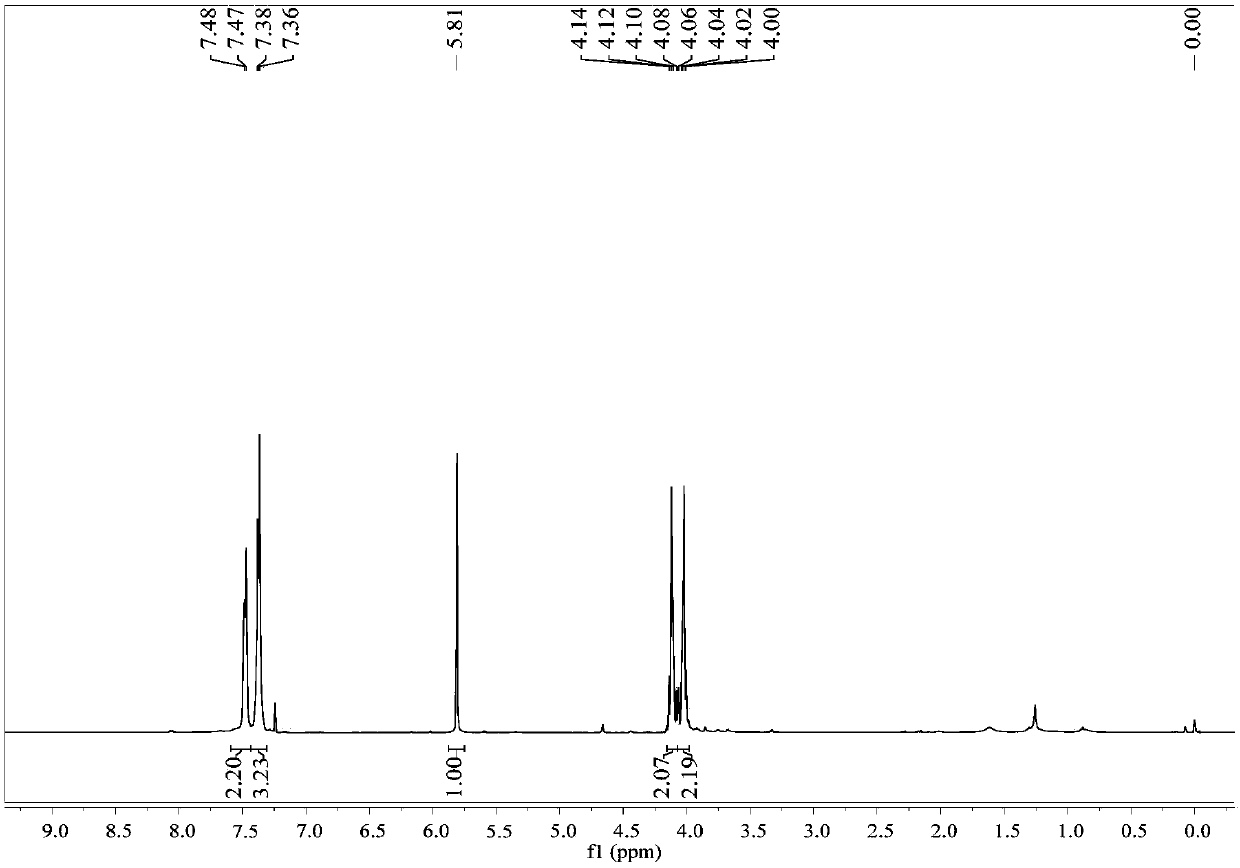
[0025] colorless liquid; 1 H NMR (400MHz, CDCl 3 )δ7.48(d,J=5.8Hz,1H),7.37(d,J=5.8Hz,2H),5.81(s,1H),4.17–4.07(m,1H),4.07–3.97(m,1H ).
Embodiment 2
[0026] Embodiment 2, the preparation of 2-phenyl-1,3-dioxolane
[0027] Nanoporous metal gold catalyst (9.8mg, 0.05mmol), ethanol (1.5mL) and 2-(2-bromophenyl)-1,3-dioxolane (57.26mg, 0.25mmol), cesium carbonate (485.73 mg, 1.5mmol) was added to the reaction kettle, hydrogen gas (30bar) was introduced, heated and stirred, the reaction temperature was controlled at 105°C, the reaction time was controlled at 45h, and the reaction solution was extracted with water to obtain 2-phenyl-1,3-di Oxolane 33.4 mg, yield 89%.
[0028]
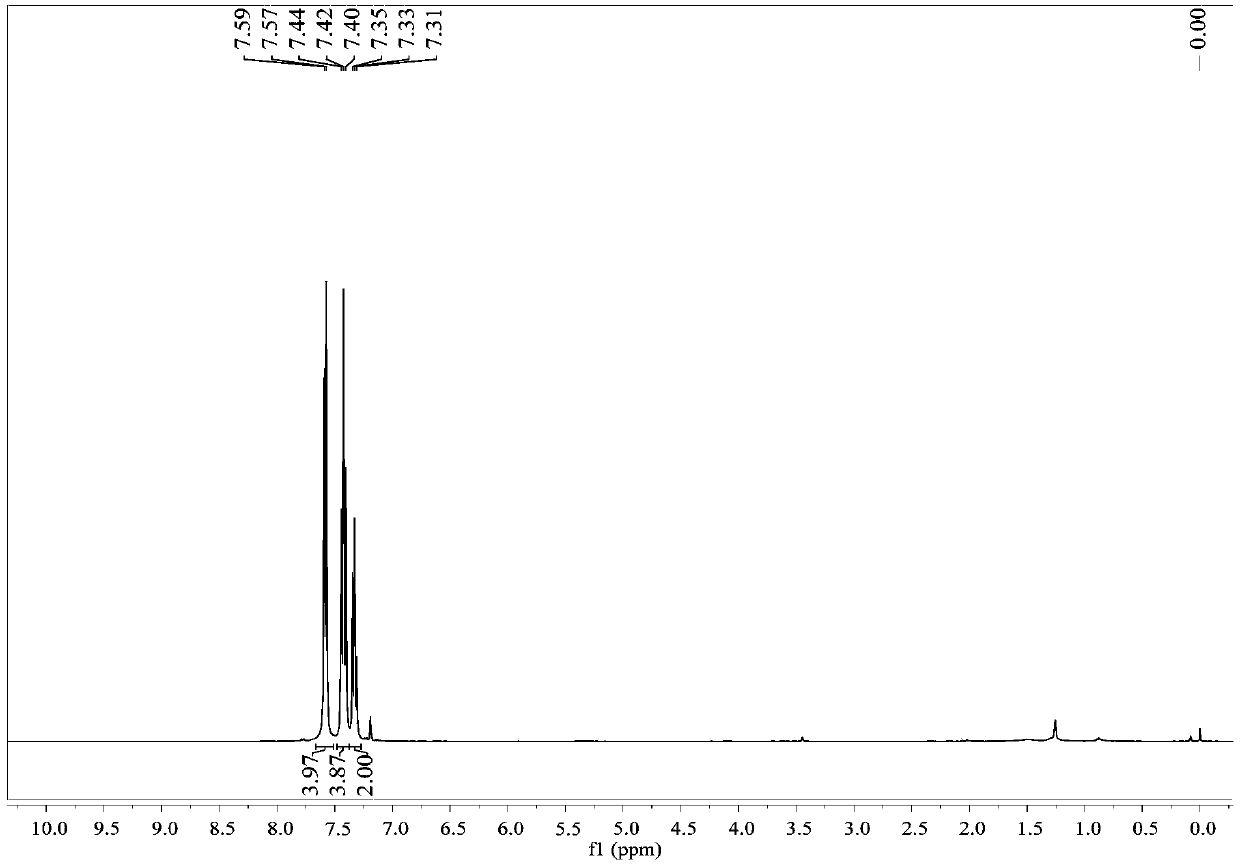
[0029] colorless liquid; 1 H NMR (400MHz, CDCl 3 )δ7.48(d,J=5.8Hz,1H),7.37(d,J=5.8Hz,2H),5.81(s,1H),4.17–4.07(m,1H),4.07–3.97(m,1H ).
Embodiment 3
[0030] Embodiment 3, the preparation of 2-phenyl-1,3-dioxolane
[0031] Nanoporous metal gold catalyst (14.7mg, 0.075mmol), methanol (2mL) and 2-(2-bromophenyl)-1,3-dioxolane (57.26mg, 0.25mmol), cesium carbonate (364.3mg , 1.125mmol) was added to the reaction kettle, hydrogen gas (25bar) was introduced, heated and stirred, the reaction temperature was controlled at 95°C, the reaction time was controlled at 50h, and the reaction solution was extracted with water to obtain 2-phenyl-1,3-diox Pentacycline 31.9mg, yield 85%.
[0032]
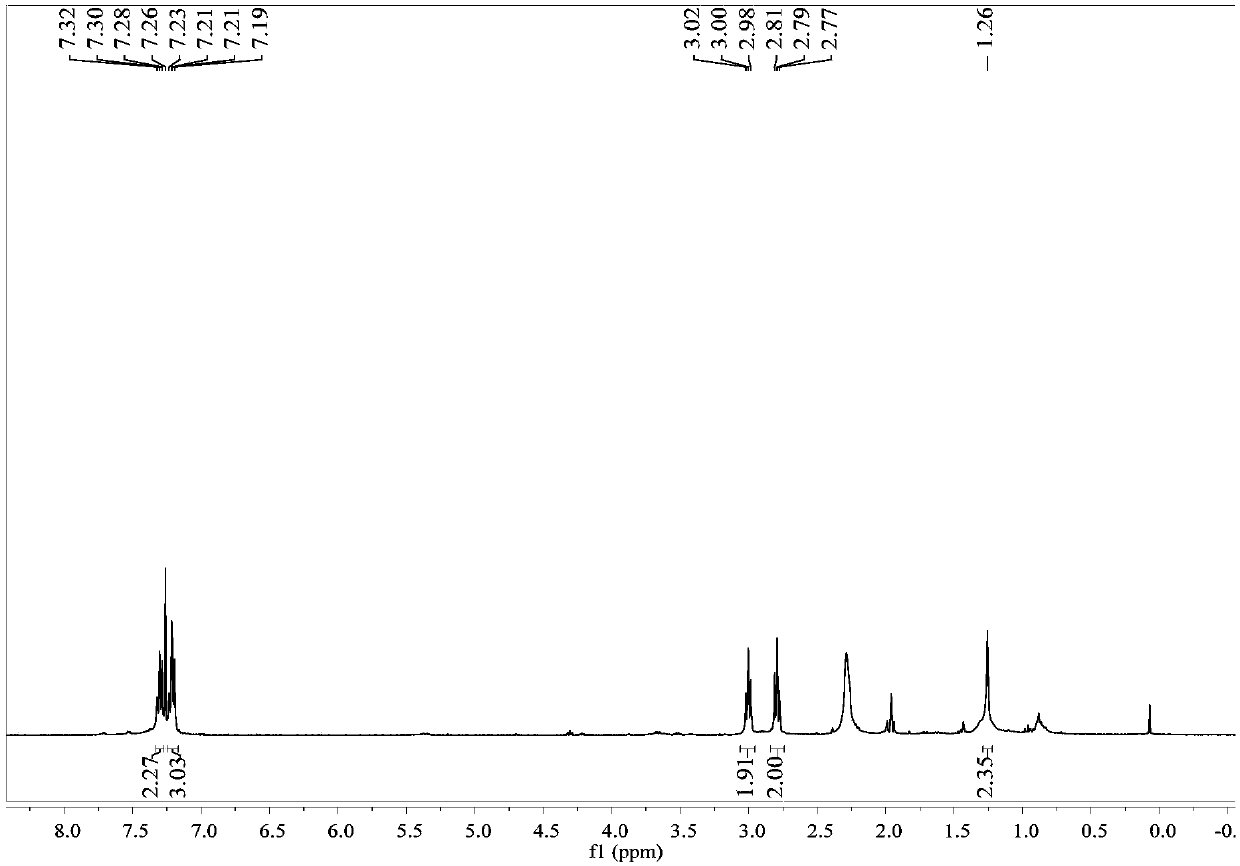
[0033] colorless liquid; 1 H NMR (400MHz, CDCl 3 )δ7.48(d,J=5.8Hz,1H),7.37(d,J=5.8Hz,2H),5.81(s,1H),4.17–4.07(m,1H),4.07–3.97(m,1H ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com