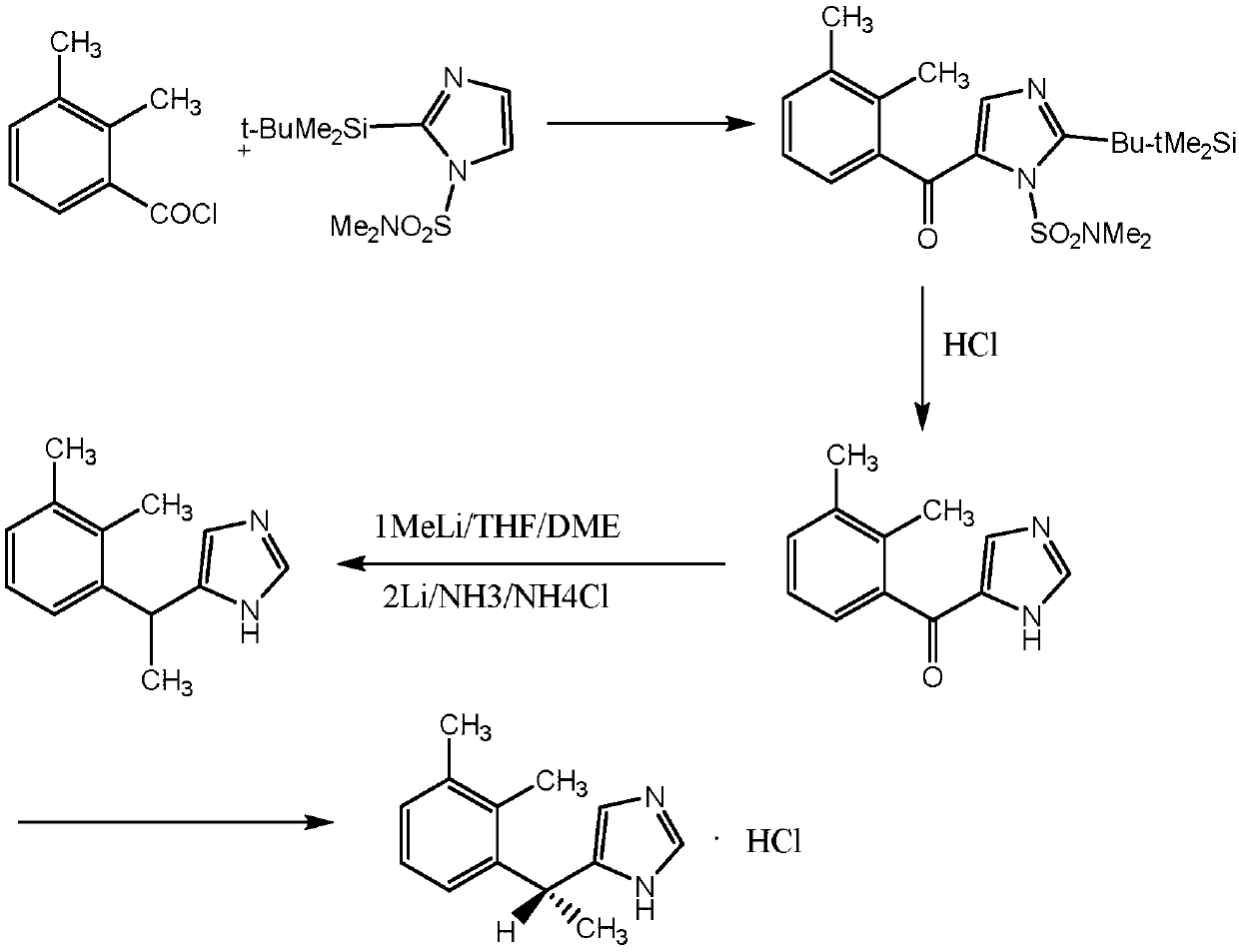
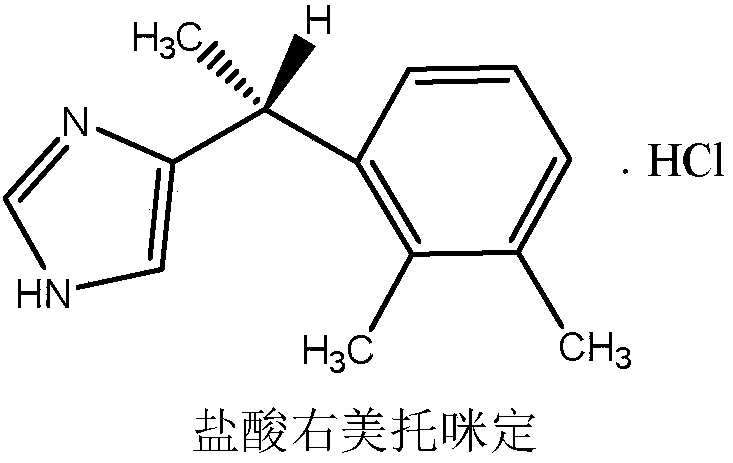
Method for synthesizing dexmedetomidine hydrochloride
A technology of dexmedetomidine hydrochloride and a synthesis method, applied in the field of synthesis of dexmedetomidine hydrochloride, can solve problems such as difficult availability, low yield, lack of starting material supply, and achieves low cost and mild reaction conditions. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Preparation of N,N-Dimethylsulfonylimidazole (Compound Ⅰ)
[0030] Add 200.0ml (1.84mol) of N,N-dimethylsulfamoyl chloride, 147.0g (2.16mol) of imidazole and 280ml of triethylamine into 2.5L of anhydrous ether under stirring, and stir at room temperature for 16h. After the reaction was completed, the reaction solution was filtered, the filter cake was washed with 1.5 L of ether, and the filtrates were combined. The filtrate was dried with anhydrous Na2SO4, concentrated in vacuo to obtain a residue, and the residue was distilled to obtain a product: 305.1 g. b.p.110°C (0.4mmHg), standing to form colorless crystals. M.p. 42-44°C. Yield: 94.75%
[0031] Preparation of 1-N,N-dimethylsulfonyl-2-(tert-butyldimethylsilyl)-5-(2,3-dimethylbenzoyl)imidazole (compound Ⅱ)
[0032] Dissolve 300.0g (1.71mol) of compound I in dry 14.0L THF, lower the temperature to -78°C under the protection of argon, add dropwise 0.96L (1.92mol) 2.0M butyllithium n-hexane solution, after 0.5h, 306...
Embodiment 2
[0045] The pre-preparation steps were the same as in Example 1, and then weighed 40.0 g (0.169 mol) of crude dexmedetomidine hydrochloride, dissolved it in 200 ml of absolute ethanol at 50° C. with stirring, and filtered the obtained solution with a G4 sand core funnel once. Add 2L ether and 0.1g recrystallization of 3-epi-sabufabin into the filtrate dropwise under stirring. After dropping, place in freezer for 5 hours, filter the precipitated crystals with suction, and dry them in vacuum to obtain 31.0g white crystals with a yield of 77.4%. mp: 153-156°C.
Embodiment 3
[0047] The pre-preparation steps were the same as in Example 1, and then weighed 40.0 g (0.169 mol) of crude dexmedetomidine hydrochloride, dissolved it in 200 ml of absolute ethanol at 50° C. with stirring, and filtered the obtained solution with a G4 sand core funnel once. Add 2L ether and 0.1g recrystallization of sabubufabin dropwise to the filtrate under stirring, after dropping, place in freezer for 5 hours, filter the precipitated crystals with suction, and dry them in vacuum to obtain 38.7g white crystals with a yield of 96.8%. mp: 153-156°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com