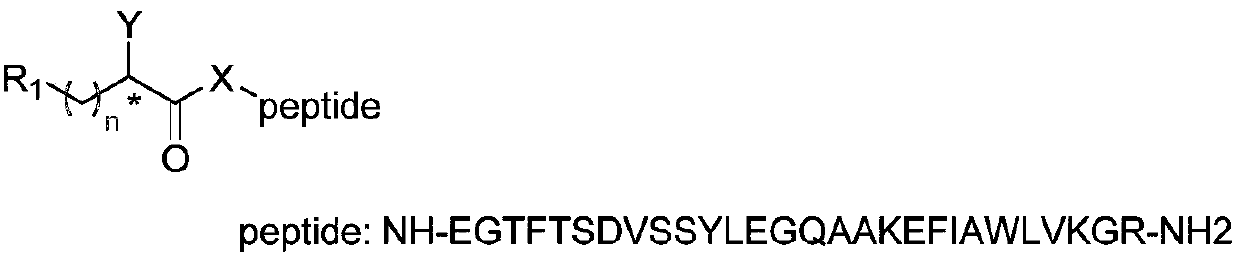Polypeptide derivative combining DDP-4 inhibiting and GLP1R activating activities and preparation and application thereof
A compound, C1-C6 technology, applied in the field of medicine, can solve problems such as unfavorable drugs and complex structure, and achieve the effect of high inhibitory activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
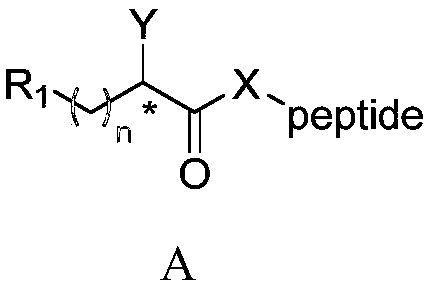
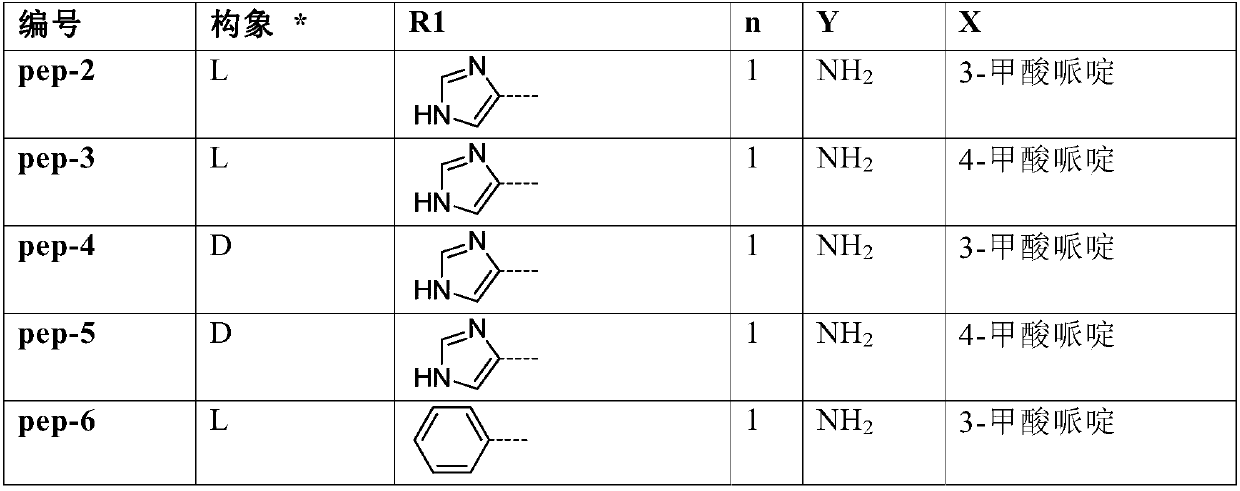
[0145] 1 equivalent of N-tert-butoxycarbonyl (Boc) amino acid, 1 equivalent of the corresponding ethyl piperidine carboxylate, 1.2 equivalents of 2-(7-oxybenzotriazole)-N,N,N',N '-Tetramethyluronium hexafluorophosphate (HATU) and 2 equivalents of triethylamine were dissolved in dichloromethane, stirred at room temperature for 8 hours, and the reaction was detected by thin layer chromatography (TLC). After the reaction was completed, dichloromethane and 1N hydrochloric acid aqueous solution were added, the organic phase was separated, washed three times with water, dried and concentrated, and the dichloromethane was evaporated in vacuo to obtain a crude product. The crude product was separated and purified by silica gel chromatography to obtain ethyl N-Boc aminopiperidinecarboxylate amide. The intermediate was dissolved in dichloromethane, and trifluoroacetic acid was added to make trifluoroacetic acid:dichloromethane=1:10. The reaction was stirred at room temperature for 1 ho...
Embodiment 2
[0157] Example 2 Determination of Inhibitory Activity of Compounds on Human DPP-4 Enzyme in Vitro
[0158] Each compound was dissolved using DMSO (final concentration 50 ).
Embodiment 3
[0159] Example 3 Determination of Inhibitory Activity of Compounds on Human GLP1R Receptor in Vitro
[0160] Preparation of reagents:
[0161] Prepare Hanks equilibration buffer using raw materials and reagents listed in Table 3:
[0162] 1. Cremophor EL (3% PBS solution)
[0163] 2. Sulfinpyrazone (250mM DMSO solution)
[0164] 3. Fluo-4, AM (2mM DMSO solution)
[0165] 4. Serotonin (10mM DMSO solution)
[0166] HBSS calcium ion buffer: BSA 0.1%, sulfinpyrazone 250μM
[0167] Table 3. Materials and reagents required for GLP1R agonistic activity test
[0168]
[0169]
[0170] Experimental operation:
[0171] 1) Inoculate cells with high expression of GLP1R into 96 empty plates with black transparent bottom, and the cell density is about 30,000cells / 100μL / well. The 96-well plate was placed at 37°C and incubated overnight.
[0172] 2) Conduct the experiment the next day. The calcium ion buffer used for staining needs to be prepared immediately. The method is as...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com