Joint thermodynamic model-COMSO-UNIFAC
A thermodynamic model and modular calculation technology, applied in the fields of informatics, computational theoretical chemistry, instruments, etc., can solve the problems of large manpower, material resources and financial resources
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
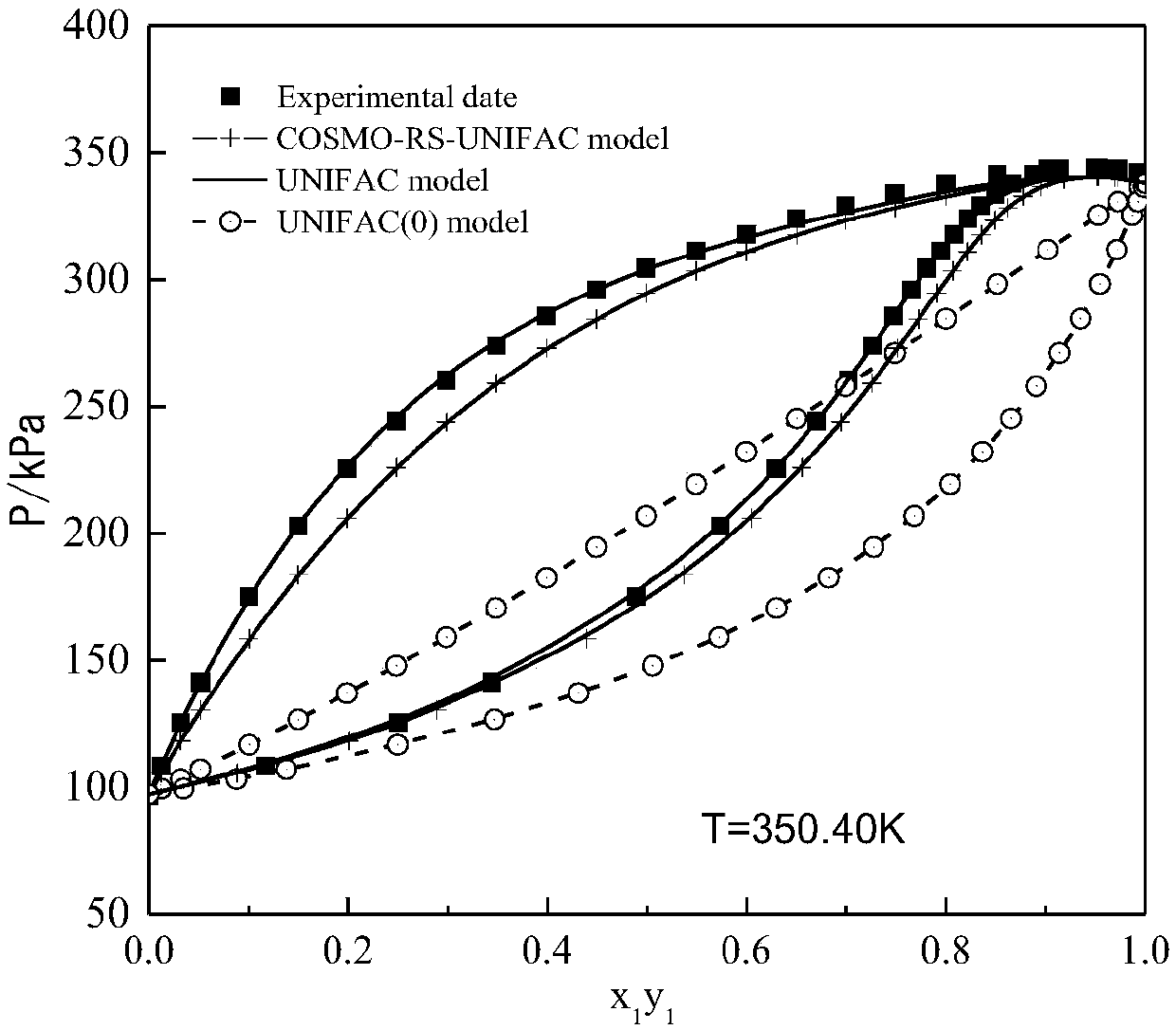
Image
Examples
Embodiment 1
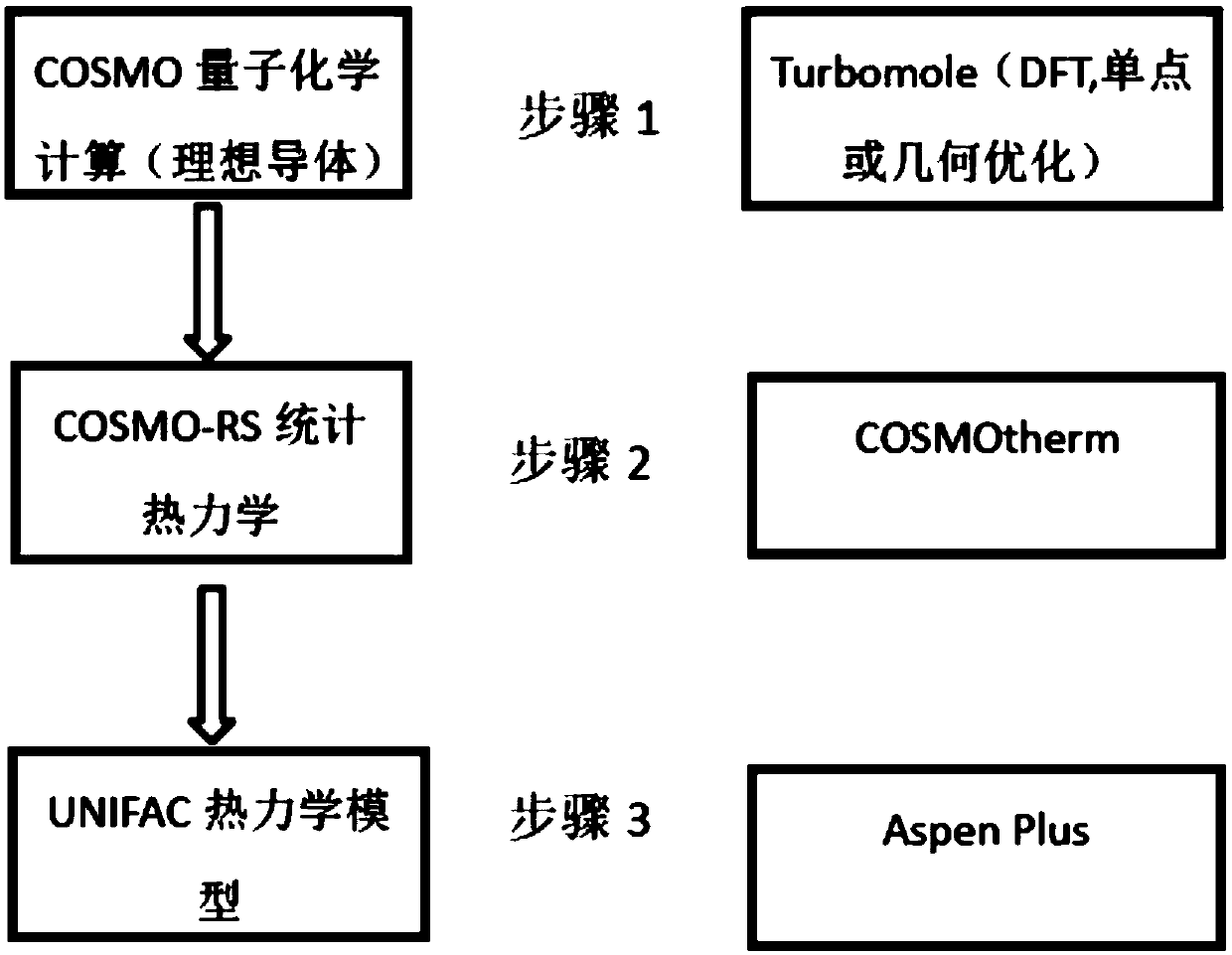
[0020] (1) Taking perfluorohexane and acetone as examples, as attached figure 1 The flow chart shown in the figure, using the single-point algorithm of density functional theory to calculate the energy of the structurally stable molecular conformation, and perform geometric optimization on the molecular conformation stabilized by perfluorohexane and acetone to form a COSMO file.
[0021] (2) Import the COSMO file generated in step (1) into the database of COSMOtherm software, and calculate the infinite dilution condition of acetone in perfluorohexane every 2°C within the range of 0-120°C through the COSMO-RS model of COSMOtherm software Under (the mass fraction of acetone A as solute in the solvent of perfluorohexane B is not more than 10 -10 ) activity coefficient.
[0022] (3) Bring the above obtained activity coefficient under the condition of infinite dilution into the UNIFAC model for parameter correlation, and finally [CF 3 ] and [CH 3 The interaction parameters betw...
Embodiment 2
[0024] (1) with methanol (CH 3 OH) and dimethylacetamide (DMAC) as examples, as attached figure 1 The flow chart shown in , using the single-point algorithm of density functional theory to calculate the energy of the structurally stable molecular conformation, and perform geometric optimization on the molecular conformation stabilized by methanol and dimethylacetamide to form a COSMO file.
[0025](2) Import the COSMO file generated in step (1) into the database of COSMOtherm software, and use the COSMO-RS model of COSMOtherm software to calculate the infinite dilution of methanol in dimethylacetamide every 2°C within the range of 0-120°C Under conditions (the mass fraction of methanol as solute in this solvent of dimethylacetamide is no more than 10 ^-9 ) activity coefficient.
[0026] (3) Bring the above obtained activity coefficient under infinite dilution conditions into the UNIFAC model for parameter correlation, and finally get the interaction parameters between [OH] ...
Embodiment 3
[0028] (1) Take methyl sulfide (C 2 h 6 S) and ethanol (C 2 h 5 OH) as an example, as attached figure 1 As shown in the flow chart, the single-point algorithm of density functional theory is used to calculate the energy of the structurally stable molecular conformation, and the calculation of the stable molecular conformation of dimethyl sulfide and ethanol is performed to form a COSMO file.
[0029] (2) Import the COSMO file generated in step (1) into the database of the COSMOtherm software, and use the COSMO-RS model of the COSMOtherm software to calculate every 2°C of methyl sulfide in the range of 0-120°C under the condition of infinite dilution in ethanol (the mass fraction of methyl sulfide as a solute in ethanol is no more than 10 ^-9 ) activity coefficient.
[0030] (3) Bring the above obtained activity coefficient under infinite dilution into the UNIFAC model for parameter correlation. The UNIFAC model can use the group contribution method to split dimethyl sulf...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com