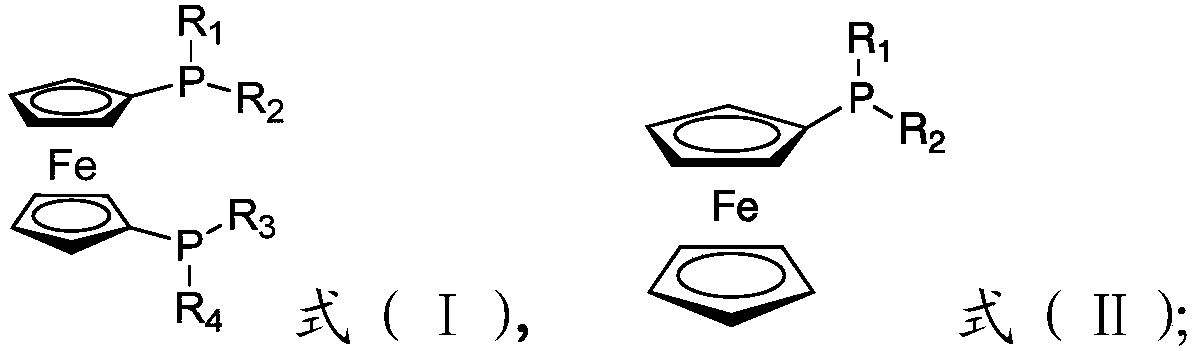
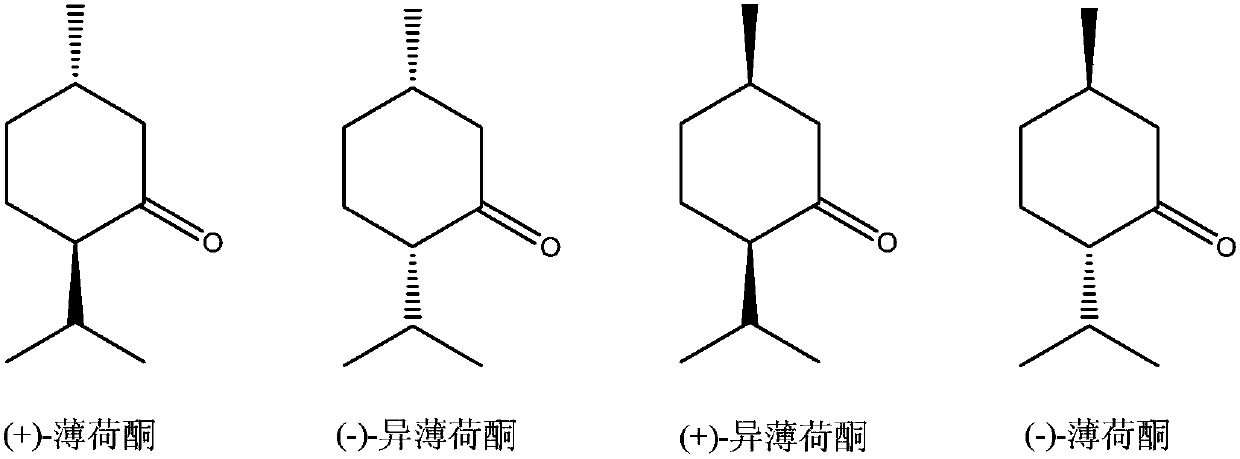
Method for preparing L-menthone
A technology of menthone and isopulegol, which is applied in the field of selectively catalyzing L-isopulegol to prepare L-menthone, can solve the problems of limited turnover increase, adverse environmental impact, and increased process complexity, achieving increased The effect of longevity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
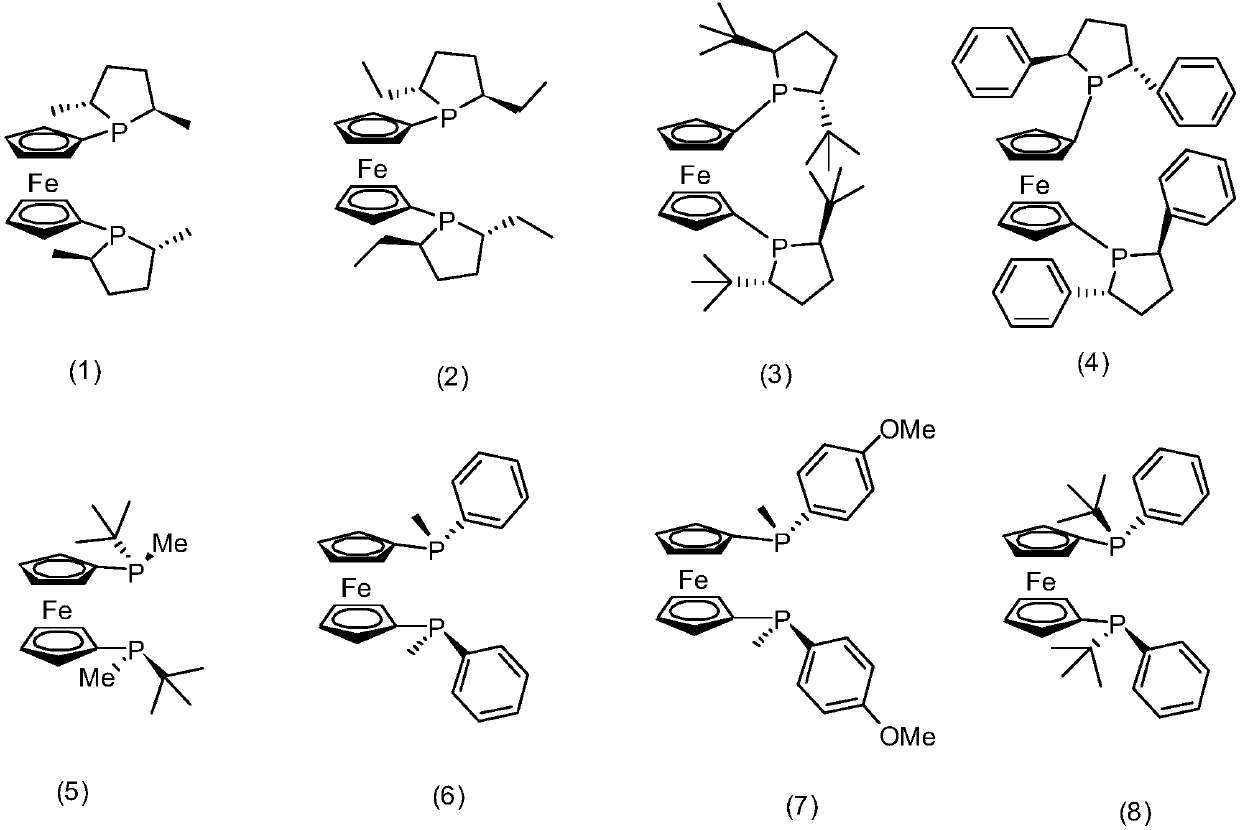
[0059] 41.4mg (0.1mmol) bisphosphine ligand (chiral phosphine ligand compound (1)), 20.7mg (0.1mmol) RuCl 3 Dissolved in 15mL toluene, and transferred to a 50mL reaction kettle, 30.44g (0.2mol) racemic isopulegol (ee=0%, L-isopulegol / D-isopulegol=1:1 (mol)) was injected into the reaction kettle, and the pressure was adjusted to 1 bar after passing through the gas in the nitrogen replacement kettle for three times. Start stirring and react at 150°C for 10 hours. The conversion rate of L-isopulegol is 99.9% as measured by gas chromatography, and the conversion of D-isopulegol does not occur. The product is L-menthone with an optical purity of 99ee%. , no isomenthone is generated, and the yield of L-menthone is 99.8% based on the L-isopulegol in the raw material.
Embodiment 2
[0061] 82.8mg (0.2mmol) bisphosphine ligand (chiral phosphine ligand compound (1)), 41.4mg RuCl 3 (0.2mmol) was dissolved in 15mL of toluene, and transferred to a 50mL reaction kettle, 15.22g L-isopulegol (ee=98%) was injected into the reaction kettle, and the pressure was adjusted to 50 after passing through the gas in the nitrogen replacement kettle for three times. bar. Start stirring and react at 150°C for 10 hours. The conversion rate of L-isopulegol is 99.9% as measured by gas chromatography, and the conversion of D-isopulegol does not occur. The product is L-menthone with an optical purity of 99ee%. , no isomenthone is generated, and the yield of L-menthone is 99.8% based on the L-isopulegol in the raw material.
Embodiment 3
[0063] 165.6mg (0.4mmol) bisphosphine ligand (chiral phosphine ligand compound (1)), 20.7mg (0.1mmol) RuCl 3 Dissolve in 15mL toluene and transfer to a 50mL reactor, inject 30.44g (0.2mol) L-isopulegol (ee=50%) into the reactor, pass through nitrogen to replace the gas in the reactor for three times and then adjust the pressure to 1 bar. Start stirring and react at 150°C for 10 hours. The conversion rate of L-isopulegol is 99.9% as measured by gas chromatography, and the conversion of D-isopulegol does not occur. The product is L-menthone with an optical purity of 99ee%. , no isomenthone is generated, and the yield of L-menthone is 99.8% based on the L-isopulegol in the raw material.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com