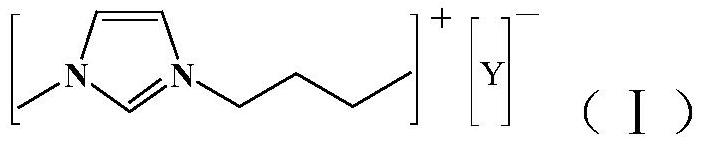
A kind of imidazole ionic liquid and its preparation and application in vitamin e acetate synthesis
A technology of ionic liquids and imidazoles, which is applied in the field of imidazole ionic liquids and their preparation and in the synthesis of vitamin E acetate, which can solve the problems of large environmental pollution, difficult catalyst recovery, and high catalyst prices
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Weigh 55g (0.25mol) of 1-butyl-3-methylimidazole bromide and 34g (0.25mol) of zinc chloride and add them to a reaction flask with a reflux condenser and nitrogen protection, and use an appropriate amount of n-heptane to make Solvent, under constant temperature condition of 105°C, magnetically stirred for 12h. After the reaction was completed, the n-heptane was removed by rotary evaporation under reduced pressure, and the light yellow viscous transparent ionic liquid bromide-1-butyl-3-methylimidazolium zinc chloride salt ionic liquid was obtained after vacuum drying, with a yield of 96%.
[0033] Product characterization: 1 H NMR (500MHz, DMSO): δ0.80(t, 3H, J=7.3Hz), 1.19(m, 2H), 1.70(m, 2H), 3.80(s, 3H), 4.10(t, 2H, J =7.2Hz),7.55(s,1H),7.61(s,1H),8.92(s,1H).
Embodiment 2
[0035] Weigh 55g (0.25mol) of 1-butyl-3-methylimidazole bromide and 56g (0.25mol) of zinc bromide into a reaction flask equipped with a reflux condenser and nitrogen protection, and use an appropriate amount of n-heptane to make Solvent, under constant temperature condition of 105°C, magnetically stirred for 12h. After the reaction was completed, the n-heptane was removed by rotary evaporation under reduced pressure, and the light yellow viscous transparent ionic liquid bromide-1-butyl-3-methylimidazolium zinc bromide ionic liquid was obtained after vacuum drying with a yield of 98%.
[0036] Product characterization: 1 H NMR (500MHz, DMSO): δ0.80(t, 3H, J=7.3Hz), 1.19(m, 2H), 1.70(m, 2H), 3.80(s, 3H), 4.10(t, 2H, J =7.2Hz),7.55(s,1H),7.61(s,1H),8.92(s,1H).
Embodiment 3
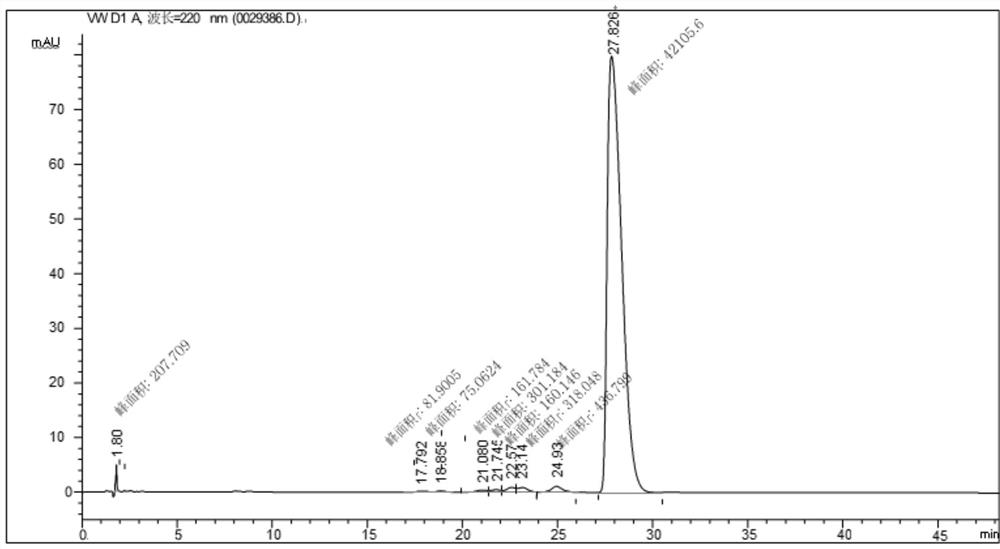
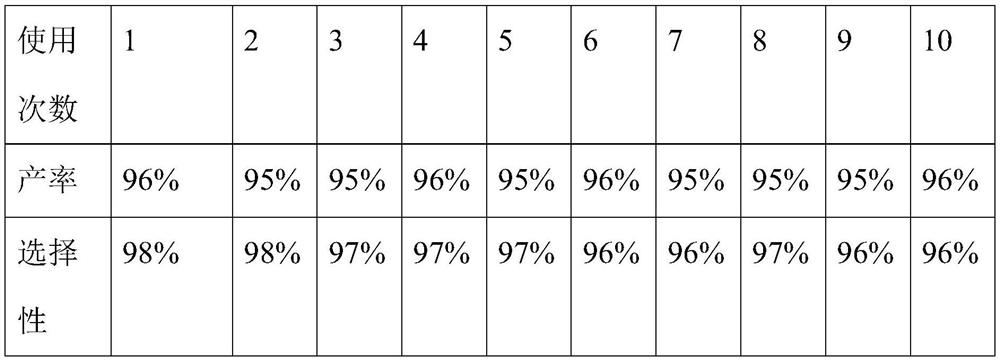
[0038] Weigh 2.5g bromide-1-butyl-3-methylimidazolium zinc chloride salt ionic liquid and 10.00g 2,3,5-trimethylhydroquinone diacetate into the reaction bottle, then add 45mL petroleum ether As a solvent, an oil bath was used to heat the temperature of the reaction liquid to 70° C., and with magnetic stirring, 12.55 g of isophytic alcohol was slowly added dropwise with a constant pressure funnel, and the reaction time was controlled at 4 hours. After the reaction, the reaction solution was left to stand, and the catalyst and vitamin E were separated, then 4.32g of acetic anhydride was weighed and added to the product, and the oil bath was 120°C, and the reaction was carried out for 3 hours to obtain the final product vitamin E acetate with a yield of 80%. , the selectivity is 88%, and the catalyst separated by cooling and standing can be recycled.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com