Bifidobacterium longum capable of relieving atopic dermatitis and application thereof
A technology for atopic dermatitis and Bifidobacterium longum, applied in the fields of microbiology and medicine, can solve problems such as poor tolerance of patients, difficulty in AD treatment, adverse reactions, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Embodiment 1: Screening and strain identification of Bifidobacterium longum
[0056] 1. Screening
[0057] Taking healthy human feces from Hangzhou, Zhejiang as the sample, the sample was pretreated and stored in a -80°C refrigerator in about 20% glycerin. After taking out and thawing, mix the sample and add 0.5mL sample to 4.5mL. 0.9% normal saline containing 0.05% cysteine was used for gradient dilution, and the appropriate gradient dilution was selected and spread on the MRS solid medium added with 0.05% cysteine, cultured at 37°C for 48 hours, and typical colonies were picked Streak on MRS solid medium for purification, pick a single colony and transfer to MRS liquid medium (containing 0.05% cysteine) for enrichment, and preserve in 30% glycerol to obtain strain CCFM1029, strain A1 and strain A2.
[0058] 2. Identification
[0059] The genome of CCFM1029, A1, A2 was extracted, and the 16S rDNA of CCFM1029, A1, A2 was amplified and sequenced (by Yingwei Jieji (Sh...
Embodiment 2
[0060] Embodiment 2: the cultivation of Bifidobacterium longum
[0061] After inserting Bifidobacterium longum (Bifidobacterium longum) CCFM1029 into MRS solid medium (containing 0.05% cysteine) and culturing at 37°C for 48 hours, the colonies were observed, and the colonies were found to be convex to cushion-like, with complete, soft edges. Moist, white and glossy.
[0062] Insert Bifidobacterium longum CCFM1029 into MRS liquid medium (containing 0.05% cysteine) and culture it anaerobically at 37°C for 24 h, then transfer to fresh MRS liquid medium (containing 0.05% cysteine). acid), cultivated under the same conditions for 24 hours, centrifuged the cells at 6000 g for 15 min, washed the cells with 0.9% normal saline and centrifuged again at 6000 g for 10 min to obtain the cells, resuspended with 30% sucrose solution, and frozen at -80°C until use.
Embodiment 3
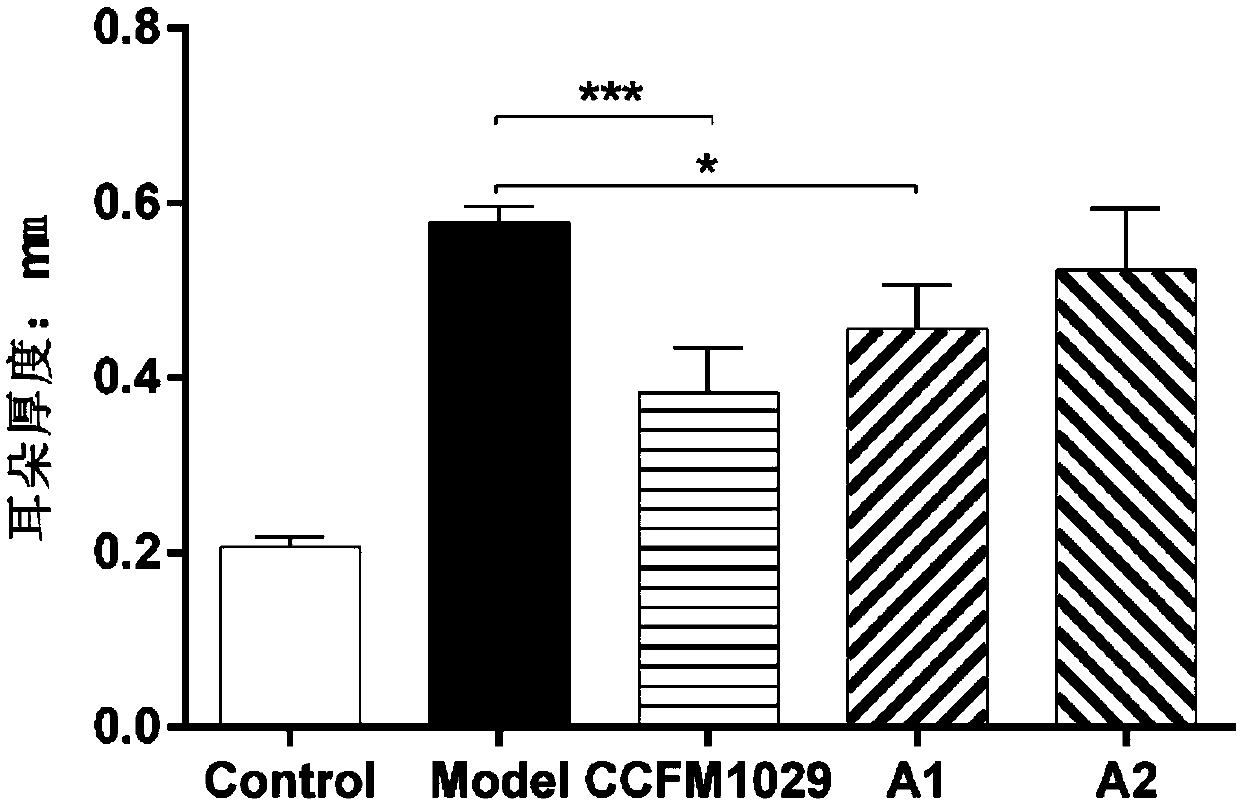
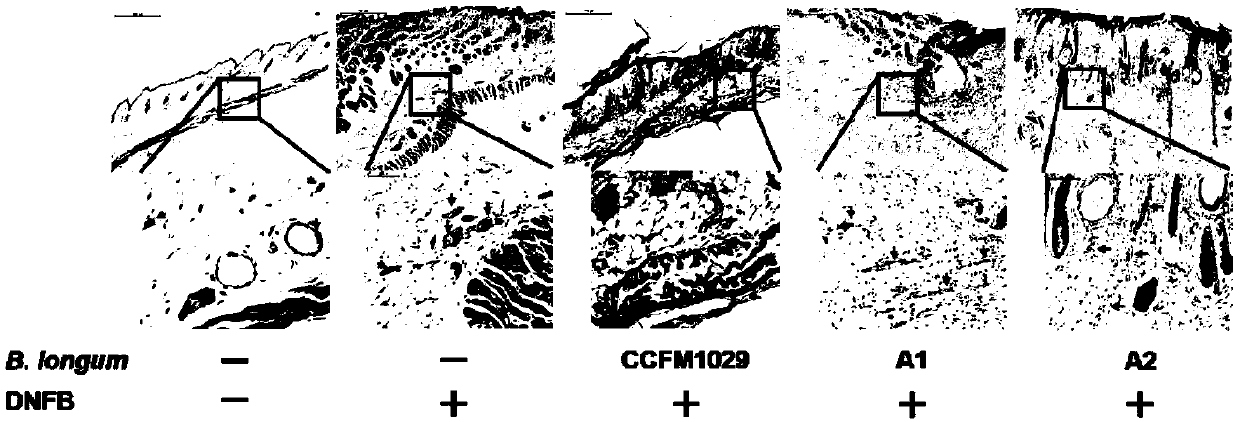
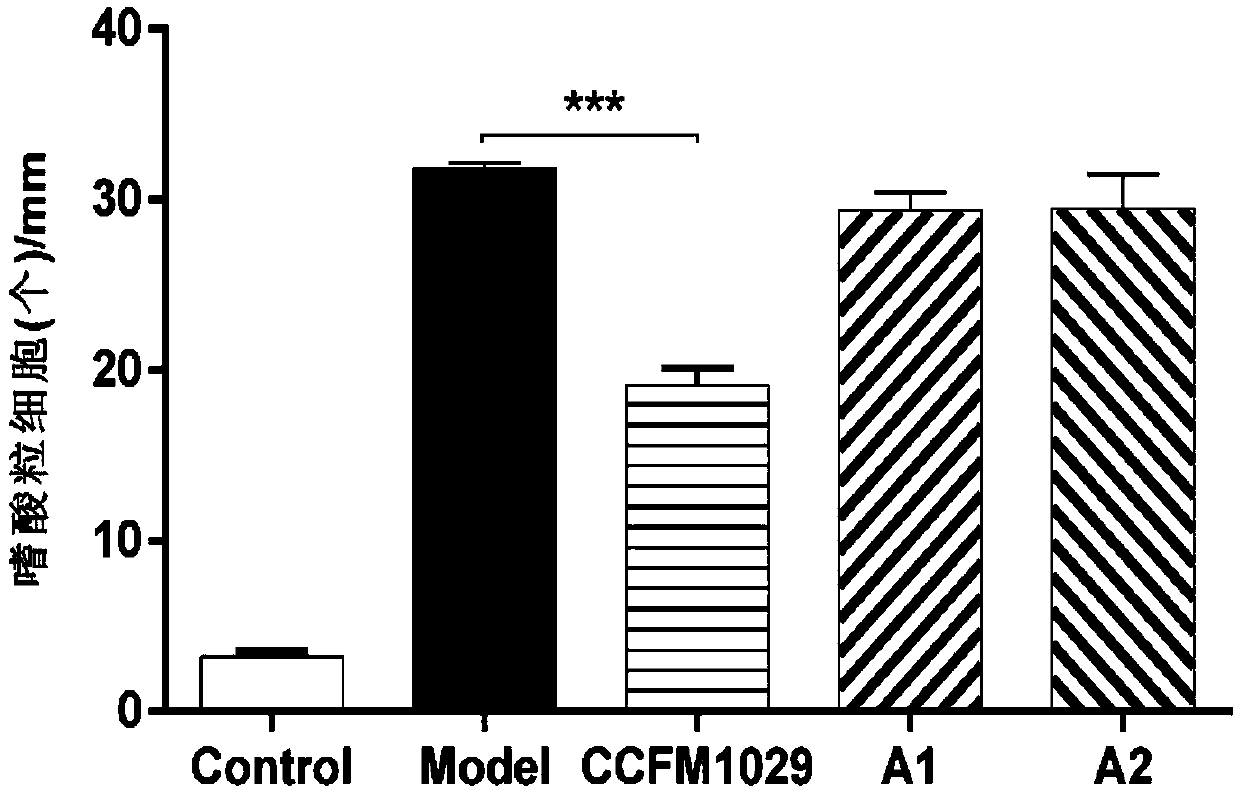
[0063] Example 3: Effects of different Bifidobacterium longum on ear thickness of mice with atopic dermatitis
[0064] Take 50 healthy female C57BL / 6 mice with a body weight of 18-20g, and divide them into 5 groups randomly, 10 mice in each group. The 5 groups are: blank group (Control), administration of 2,4-dinitrofluorobenzene (2,4-dinitrofluorobenzene, DNFB) model group (Model), the CCFM1029 group administered with Bifidobacterium longum CCFM1029, the A1 group administered with Bifidobacterium longum A1, the A2 group administered with Bifidobacterium longum A2, wherein , CCFM1029 group, A1 group, A2 group are treatment groups.
[0065] The experiment lasted for four weeks: the first week was the adaptation period of the mice; the second week began to gavage the mice until the end of the experiment. The total amount of live bacteria is 1×10 9 CFU) / head / time for intragastric administration, the blank group and the model group were not intervened with bacterial solution, an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com