Application of bacillus coagulans in the preparation of medicines for preventing and/or treating allergic reactions
A technology for Bacillus coagulans and allergic reaction, which is applied in the field of functional food composition and preparation thereof, and can solve the problems of easily causing allergic reaction and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
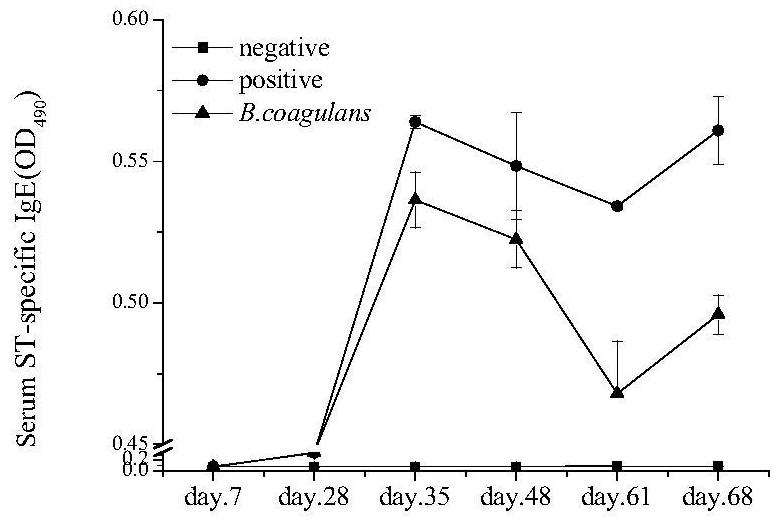
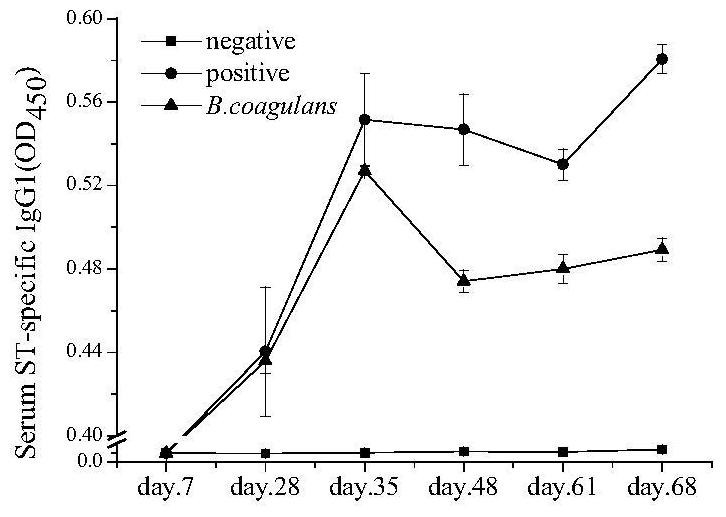
[0032] In the present invention, the specific IgE and IgG levels in serum, histamine content, cytokine content in cell supernatant, cell RNA expression and other indicators are used to evaluate the regulatory effect of bacillus coagulans on allergic reaction.
[0033] 1.1 Establishment of sensitized animal model
[0034] Experimental animals: detergent female Balb / c mice, 6 weeks old, weighing 18±2g, purchased from the Experimental Animal Center of Hangzhou Normal University, fed for two consecutive generations with a special feed that does not contain shrimp protein. The temperature of the rat room is 20±2°C, the humidity is 50±10%, and the day and night cycle is 12 hours to ensure ventilation. The rat cage is cleaned and disinfected twice a week. Using the method of random grouping, set up the blank control group, the allergy group, and the bacteria experiment group respectively, with 8 mice in each group.
[0035] Strain culture: Bacillus coagulans with the preservation numb...
Embodiment 2
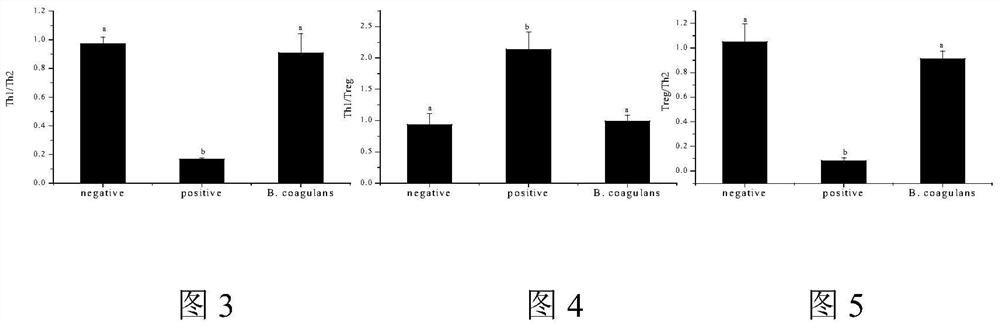
[0055] This example illustrates the effect of Bacillus coagulans of CCTCC NO: M 2013193 on Treg cells, mast cells and B cells.
[0056] 2.1 Sources of Treg cells, B cells and mast cells
[0057] Take 6-week-old female Balb / c mice with a body weight of 18-22 g. After cervical sacrifice, the spleen was aseptically removed and ground to obtain spleen lymphocytes. The above two types of cells were sorted by flow cytometry.
[0058] P815 mast cells were purchased from Shanghai Gefan Co., Ltd., and the culture conditions were: 37°C, 5% CO 2 .
[0059] 2.2 Co-culture of Bacillus coagulans with Treg cells and Th2 cells
[0060] Adjust the concentrations of Treg cells and Th2 cells to 10 6 individual / mL. In a 6-well plate, add 1 mL each of Treg cells and Th2 cells, 100 μL of Bacillus coagulans (concentration of 10 8 CFU / mL), tropomyosin was stimulated in vitro after filter sterilization (stimulating concentration was 50 μg / mL). 37°C, 5% CO 2 Conditioned for 48h. Centrifuge at...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com