Vaccine adjuvant and application thereof and porcine reproductive and respiratory syndrome vaccine
A respiratory syndrome and vaccine adjuvant technology, which is applied in the field of vaccine adjuvants and porcine reproductive and respiratory syndrome vaccines, can solve the problems of shortening the production time of neutralizing antibodies, enhance safety and immunity, and reduce viremia , improve the effect of cellular immunity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
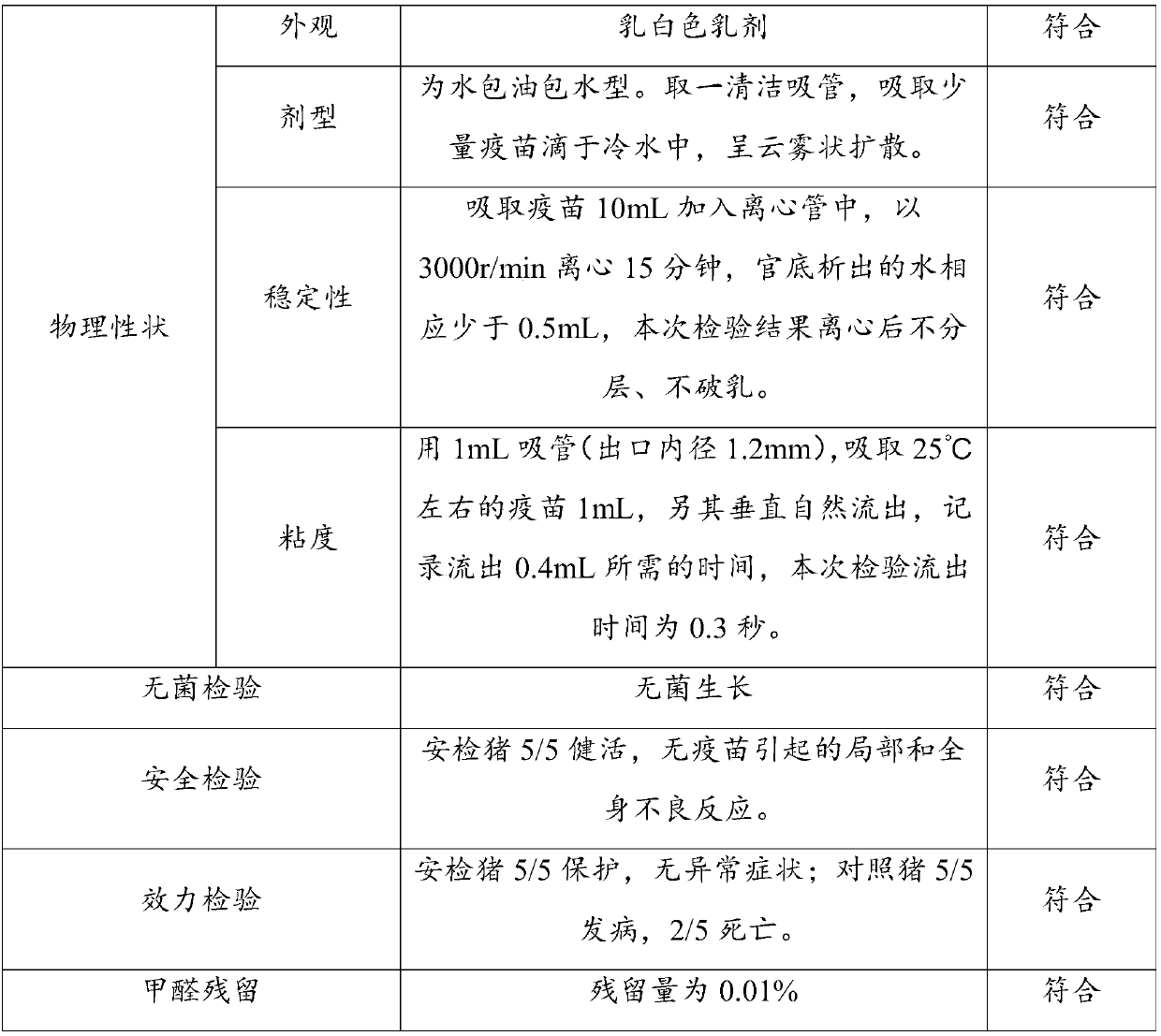
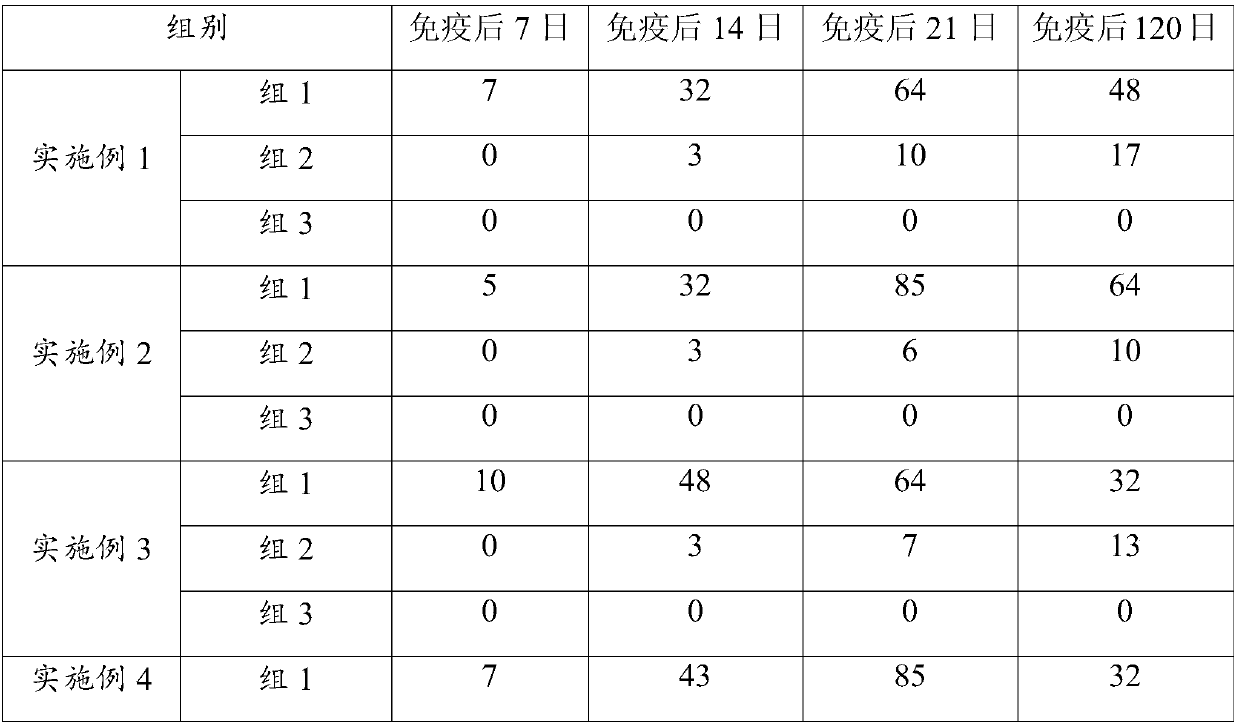
Image
Examples
Embodiment 1
[0026] A vaccine adjuvant comprising the following components: 1 mg of quillaja extract, 2 mg of TLR3 receptor effector (double-stranded RNA), 5 g of diethylaminoethyl glucose and 1 g of stabilizer (phosphate buffered saline).
[0027] The porcine reproductive and respiratory syndrome vaccine of the embodiment of the present invention is prepared by the following steps:
[0028] Weigh each component according to the above-mentioned vaccine adjuvant formula, and set aside;
[0029]Add soap tree extract, TLR3 receptor effector, diethylaminoethyl glucose and stabilizer to inactivated porcine reproductive and respiratory syndrome inactivated antigen solution in sequence, and stir evenly. The pH of the porcine reproductive and respiratory syndrome vaccine was adjusted to 6.8.
Embodiment 2
[0031] A vaccine adjuvant comprising the following components: 10 mg of quillaja extract, 5 mg of TLR3 receptor effector (double-stranded RNA), 2 g of diethylaminoethyl glucose and 3 g of stabilizer (phosphate buffered saline).
[0032] The porcine reproductive and respiratory syndrome vaccine of the embodiment of the present invention is prepared by the following steps:
[0033] Weigh each component according to the above-mentioned vaccine adjuvant formula, and set aside;
[0034] Add soap tree extract, TLR3 receptor effector, diethylaminoethyl glucose and stabilizer to inactivated porcine reproductive and respiratory syndrome inactivated antigen solution in sequence, and stir evenly. The pH of the porcine reproductive and respiratory syndrome vaccine was adjusted to 7.8.
Embodiment 3
[0036] A vaccine adjuvant comprising the following components: 5 mg of quillaja extract, 3 mg of a TLR3 receptor effector (double-stranded RNA), 4 g of diethylaminoethyl glucose and 3 g of a stabilizer (phosphate buffered saline).
[0037] The porcine reproductive and respiratory syndrome vaccine of the embodiment of the present invention is prepared by the following steps:
[0038] Weigh each component according to the above-mentioned vaccine adjuvant formula, and set aside;
[0039] Add soap tree extract, TLR3 receptor effector, diethylaminoethyl glucose and stabilizer to inactivated porcine reproductive and respiratory syndrome inactivated antigen solution in sequence, and stir evenly. The pH of the porcine reproductive and respiratory syndrome vaccine was adjusted to 7.0.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com