Fusion protein antibody, enhanced vaccine and preparation method and application thereof
A fusion protein and antibody technology, applied in the field of immunity, to achieve high expression levels, promote IFN-γ secretion, and reduce costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
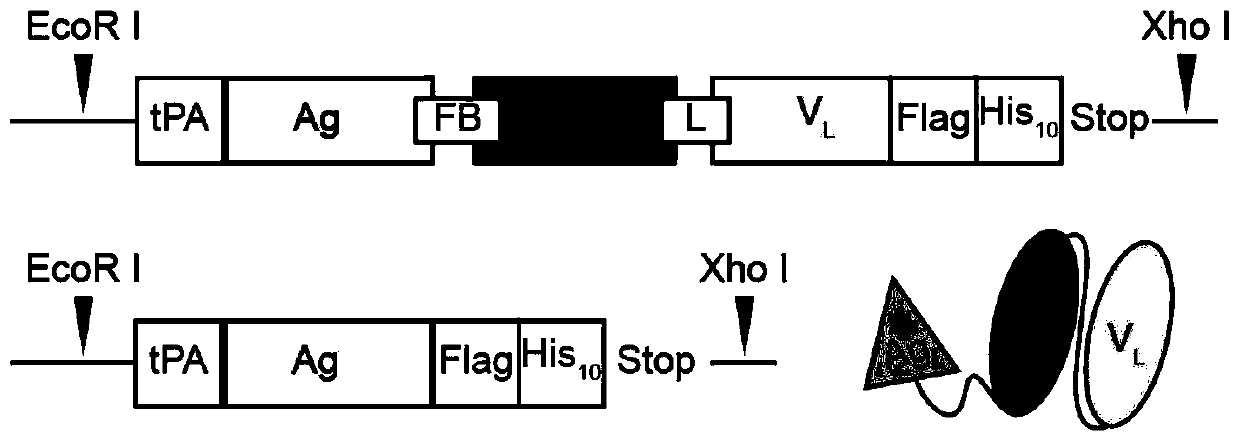
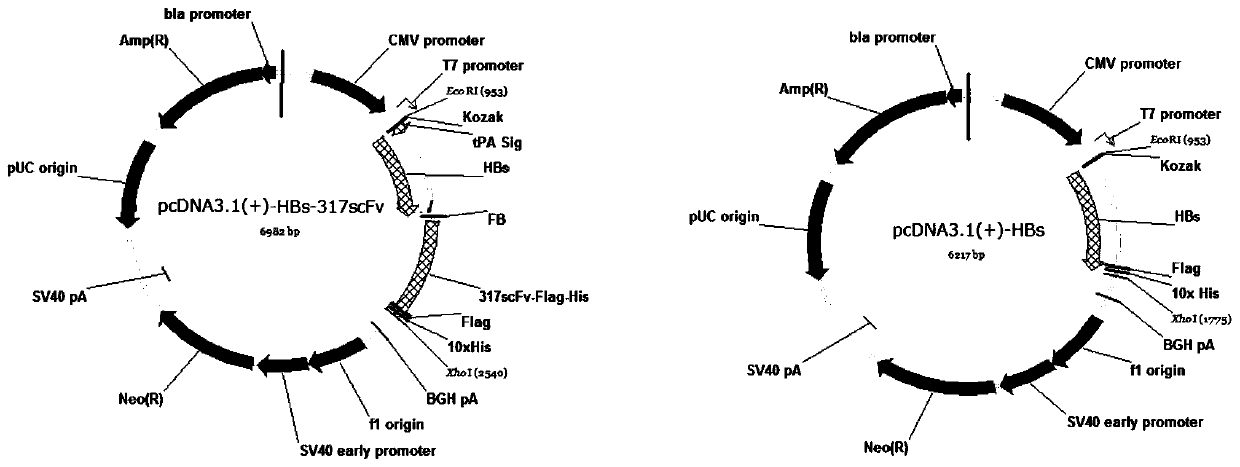
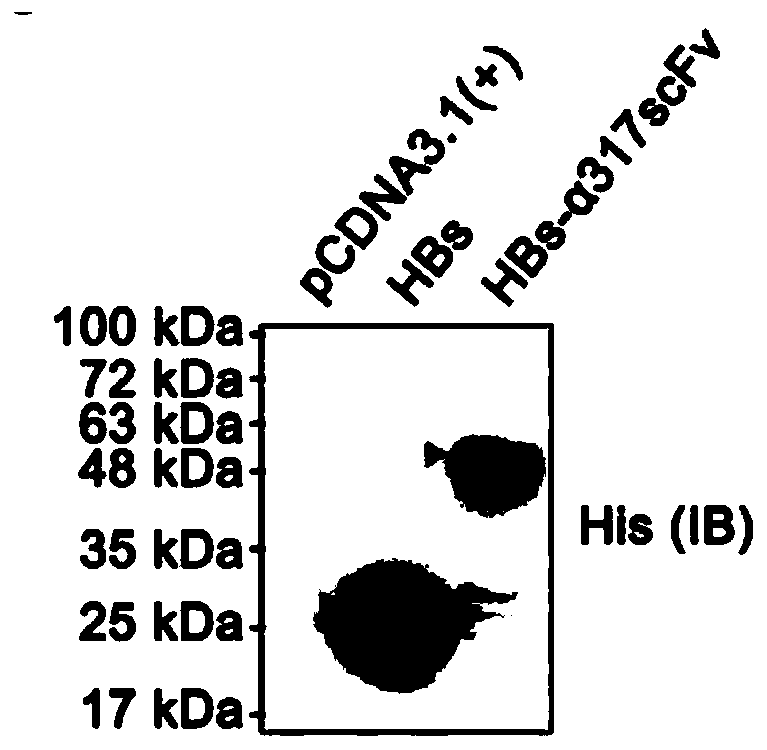
[0041] 1. Construction of eukaryotic expression vector for HBs-α317scFv recombinant protein, the plasmid structure is as attached figure 2 (left) shown.
[0042] (1) The HBs coding gene is derived from the SH1203-C2 strain genome sequence (JX661494), with a total of 678 bases from 1550 to 2227 (the nucleotide sequence is SEQ ID NO: 14, and the corresponding amino acid sequence is SEQ ID NO: 6 shown). Synthesized by Invitrogen, an auxiliary sequence is added to the 5' end (the nucleotide sequence is SEQ ID NO: 9, and the corresponding amino acid sequence is shown in SEQ ID NO: 1) to facilitate vector transformation, protein secretion and expression, and the sequence includes EcoR1 enzyme digestion Site, Kozak sequence, signal peptide (human tissue-type plasminogen activator, tPA, NM_000930, 258-323) and other elements.
[0043] (2) The α317 scFv sequence was derived from a hybridoma cell (clone number 1C12C6) secreting anti-human and mouse CD317 monoclonal antibodies constru...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com