Preparation method for intermediate of breast cancer treatment drug palbociclib
A technology for breast cancer and intermediates, applied in the field of medicine, can solve the problems of high isomer content, yield of only 80%, and undisclosed product purity, etc., and achieve the effect of high purity and simple post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
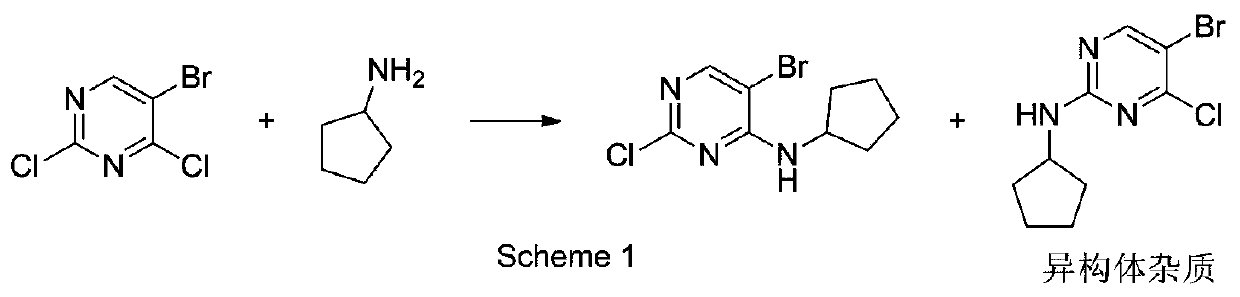
[0027] Add 5-bromo-2,4-dichloropyrimidine (10mmol, 2.27g), metal ion additive 0.5mmol, dioxane 15ml into the parallel synthesizer, stir and disperse evenly, then add cyclopentylamine (12mmol, 1.02g), stirred at room temperature after the dropwise addition, and stopped stirring when the conversion rate of the substrate 5-bromo-2,4-dichloropyrimidine was detected by HPLC no longer changed, and the substrate 5-bromo-2,4-dichloropyrimidine was counted The conversion rate of chloropyrimidine and its product 5-bromo-2-chloro-N-cyclopentyl-4-pyrimidinamine and its isomer impurity (5-bromo-4-chloro-N-cyclopentyl-2-pyrimidinamine, Abbreviated as the ratio of ISO), the results are shown in Table 1:
[0028] The influence of table 1 metal ion additive on reaction
[0029] additive
Conversion rate / %
Product / ISO
NA
96.5
84 / 13
Fe(NO 3 ) 3
93.8
82 / 12
Cu(NO 3 ) 2
98.6
86 / 12
Ni(NO 3 ) 2
90.4
78 / 12
Co(NO 3...
Embodiment 2
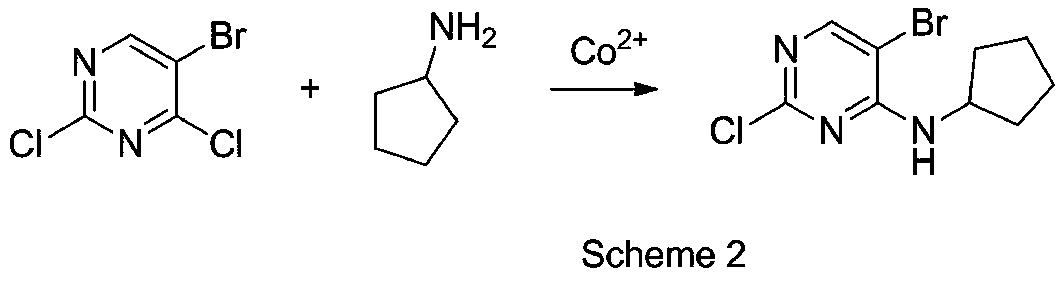
[0033] The present invention has further studied the influence of cobalt salt anion species and cobalt salt valence state on reaction, method is as follows:
[0034]Add 5-bromo-2,4-dichloropyrimidine (10mmol, 2.27g), cobalt salt 0.5mmol, dioxane 15ml into the parallel synthesizer, stir and disperse evenly, then add cyclopentylamine (12mmol, 1.02 g), stir at room temperature after the dropwise addition, stop stirring when the conversion rate of the substrate 5-bromo-2,4-dichloropyrimidine is no longer changed by HPLC, and count the substrate 5-bromo-2,4-dichloropyrimidine in the reaction system Conversion rate of pyrimidine and its product 5-bromo-2-chloro-N-cyclopentyl-4-pyrimidinamine and its isomer impurity (5-bromo-4-chloro-N-cyclopentyl-2-pyrimidinamine, abbreviated is the ratio of ISO), the results are shown in Table 2:
[0035] Table 2 The impact of the cobalt salt type on the reaction
[0036] additive
[0037] The experimental results show that the trivalen...
Embodiment 3
[0039] Selected CoSO 4 As an additive, it is used to improve the chemical reaction selectivity of the 4-position chlorine in cyclopentylamine and 5-bromo-2,4-dichloropyrimidine. In order to improve the conversion rate of substrate 5-bromo-2,4-dichloropyrimidine, combined with the present According to reports in the prior art J.Med.Chem.2014,57,3430-3449, the present invention adds an organic base to improve the conversion rate of the substrate, and the method is as follows:
[0040] Add 5-bromo-2,4-dichloropyrimidine (10mmol, 2.27g), CoSO 4 ·7H 2 O 0.5mmol, organic base 10mmol, dioxane 15ml were stirred and dispersed evenly, then cyclopentylamine (12mmol, 1.02g) was added dropwise at room temperature, stirred at room temperature after the addition, and HPLC detected substrate 5-bromo-2, Stop stirring when the conversion rate of 4-dichloropyrimidine no longer changes, and count the conversion rate of substrate 5-bromo-2,4-dichloropyrimidine and its product 5-bromo-2-chloro-N-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com