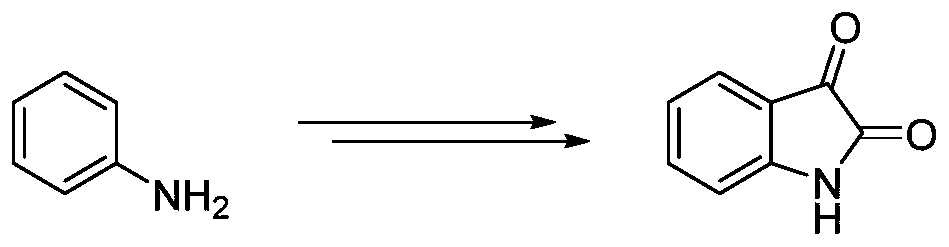
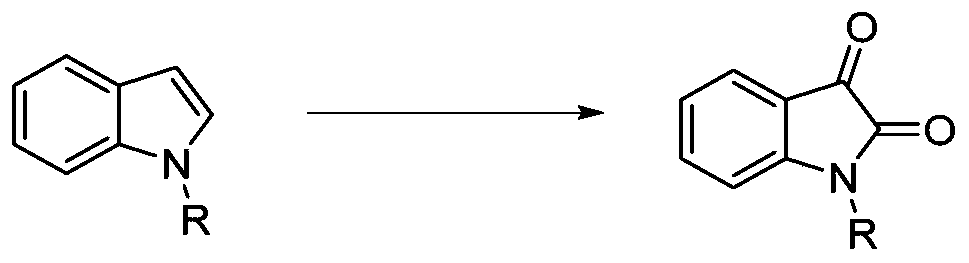
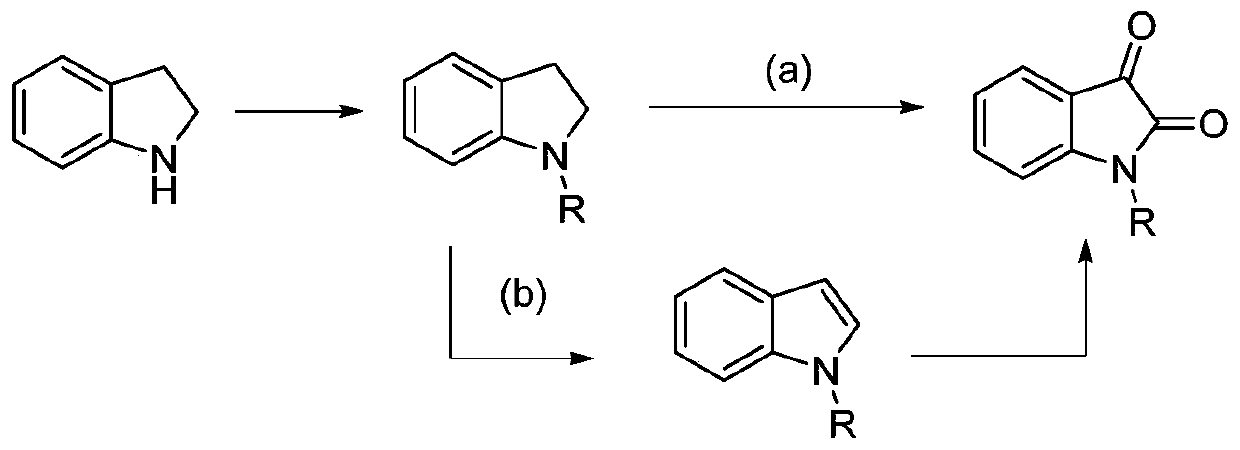
Preparation method of isatin derivatives
A derivative, isatin technology, applied in the field of preparation of isatin derivatives, can solve the problems of harsh reaction conditions, difficult to obtain substrates, etc., and achieve the effect of low reaction cost, short reaction time and simple reaction operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0022] Embodiment: First, N-methylindoline is used as a template substrate, and the reaction conditions are optimized, as shown in Table 1.
[0023] Entry
Cat.(mol%)
Oxidant
Yield(%)
1
Cu(OAc) 2 (5)
h 2 o 2 a
MeCN
15%
2
Cu(OAc) 2 (5)
K 2 S 2 o 8
MeCN
NR
3
Cu(OAc) 2 (5)
TBHP b
MeCN
25%
4
Cu(OAc) 2 (5)
TBHP b
DCE
37%
5
Cu(OAc) 2 (5)
TBHP b
DMSO
NR
6
Cu(OAc) 2 (5)
TBHP b
PhMe
10%
7
Cu(OAc) 2 (5)
TBHP b
EtOAc
5%
8
Cu(OAc) 2 (10)
TBHP b
DCE
48%
9
Cu(OAc) 2 (20)
TBHP b
DCE
60%
10
Cu(OAc) 2 (30)
TBHP b
DCE
61%
11
Cu(OAc) 2 (20)
TBHP c
DCE
88%
[0024] The reaction equation is as Figure 5 As shown, the structural formula 1a in the figure is N-methyl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com