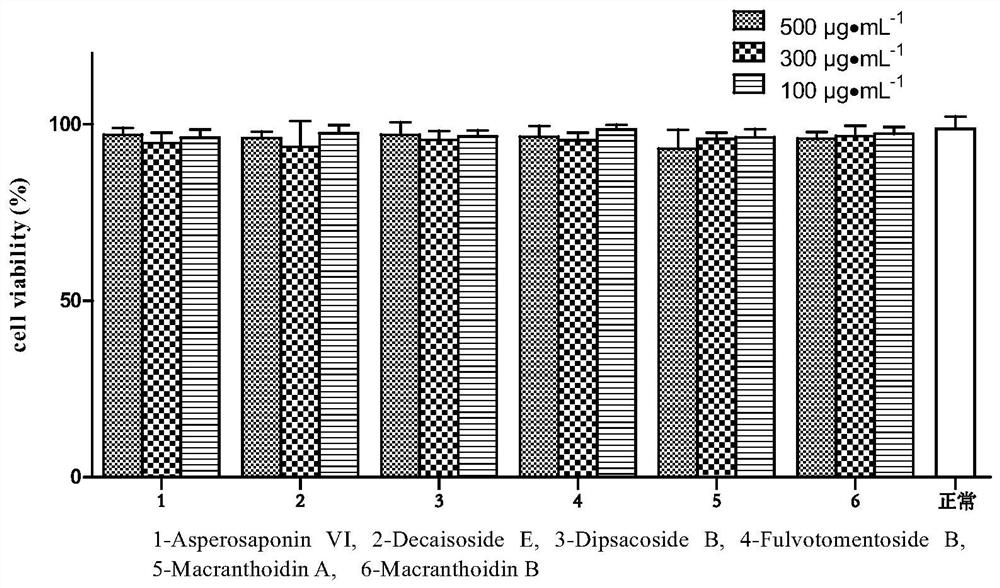
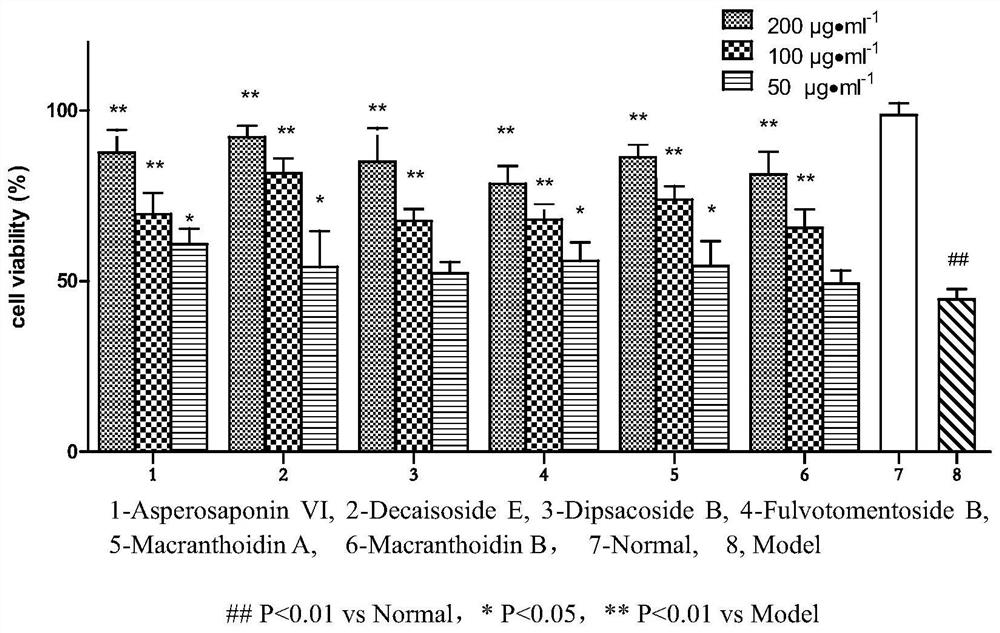
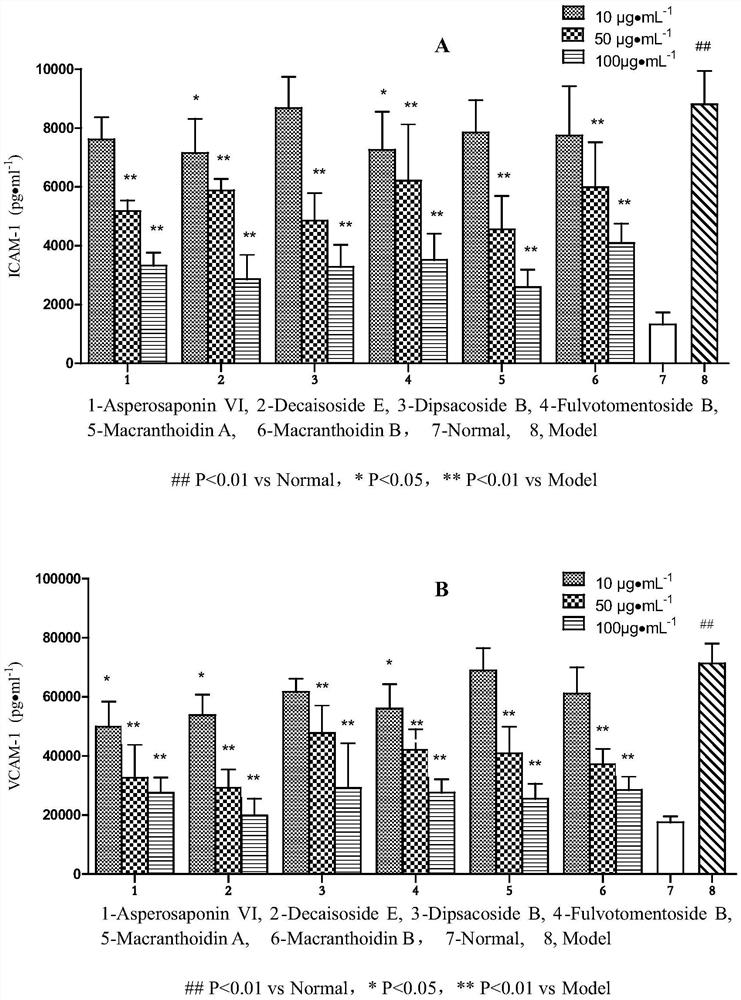
Application of hedera saponin in preparation of anti-inflammatory injury medicine of vascular endothelial cells
A technology of hederaside and acute lung injury, which is applied in the application field of disaccharide chain hederaside in the preparation of anti-vascular endothelial cell inflammatory injury drugs, and can solve the protective effect of vascular endothelial cell inflammatory injury. No reports have been reported, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] Preparation of double sugar chain hedera saponin
[0076] 10 kg of dried roots of Dipsacus chuanxiong, crushed, extracted with 95% ethanol (25L×2), combined two extracts, concentrated under reduced pressure to obtain extract. The extract was dispersed in 5L of water, filtered, and the filtrate was pretreated on the AB-8 macroporous adsorption resin column, firstly eluted with water until the color of the eluent was lighter, then eluted with 80% ethanol, collected 80% ethanol to wash Deliquification, concentration under reduced pressure to obtain the total saponins of Dipsacus chuanxiong. The total saponins of Dipsacus sativa were applied to a normal-phase silica gel chromatography column, and the gradient elution of chloroform-methanol-water system was used to obtain pure compound 1. After ESI-MS and 13 C-NMR data compared with literature data, identified as Dipsacus saponin VI (Asperosaponin VI).
[0077] Asperosaponin VI: white amorphous powder, ESI-MS: m / z927.3 ([...
Embodiment 2
[0079] Preparation of double sugar chain hedera saponin
[0080] 5 kg of dried flower buds of Lonicerae pilosula, crushed, extracted with 95% ethanol (25L×2), combined two extracts, concentrated under reduced pressure to obtain extract. The extract was dispersed in 5L of water, filtered, and the filtrate was pretreated on the AB-8 macroporous adsorption resin column, and firstly eluted with water until the color of the eluent was lighter, and then eluted with 50% methanol for 2 column volumes, Then elute with 80% ethanol, collect the 80% ethanol eluate, and concentrate under reduced pressure to obtain the total saponins of C. argentina. Take 100 g of total saponins of Lonicera tomentosa, put it on a normal-phase silica gel chromatography column, and elute with a gradient of chloroform-methanol-water system to obtain pure compounds 1 and 2, which are analyzed by ESI-MS and 13 The C-NMR data were compared with the data in the literature, and they were identified as Lonicera cin...
Embodiment 3
[0084] Preparation of double sugar chain hedera saponin
[0085] 5 kg of dried flower buds of Lonicera chrysanthemum, crushed, extracted with 95% ethanol (25L×2), combined two extracts, concentrated under reduced pressure to obtain extract. The extract was dispersed in 5L of water, filtered, the filtrate was pretreated on the AB-8 macroporous adsorption resin column, and firstly eluted with water until the color of the eluent was lighter, and then eluted with 40% methanol for 2 column volumes, Then elute with 80% ethanol, collect the 80% ethanol eluate, and concentrate under reduced pressure to obtain total saponins of flavonoids (TSF). Take 100 g of total saponins of Lonicera tomentosa, put it on a normal-phase silica gel chromatography column, and elute with the gradient of chloroform-methanol-water system to obtain pure compound 1-3, which is analyzed by ESI-MS and 13 Comparing C-NMR data with literature data, they were identified as Dipsacoside B, Decaisoside E and Fulvot...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com