A method for improving enzyme stability
An alkaline, pectinase technology, applied in the direction of enzyme stabilization, lyase, hydrolase, etc., can solve the problem of difficulty in obtaining thermostable mutants, and achieve the effect of improving stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0103] Embodiment 1: the construction of the recombinant bacterium that can express fusion enzyme
[0104] Specific steps are as follows:
[0105] Construction of recombinant bacteria that can express fusion alkaline pectinase:
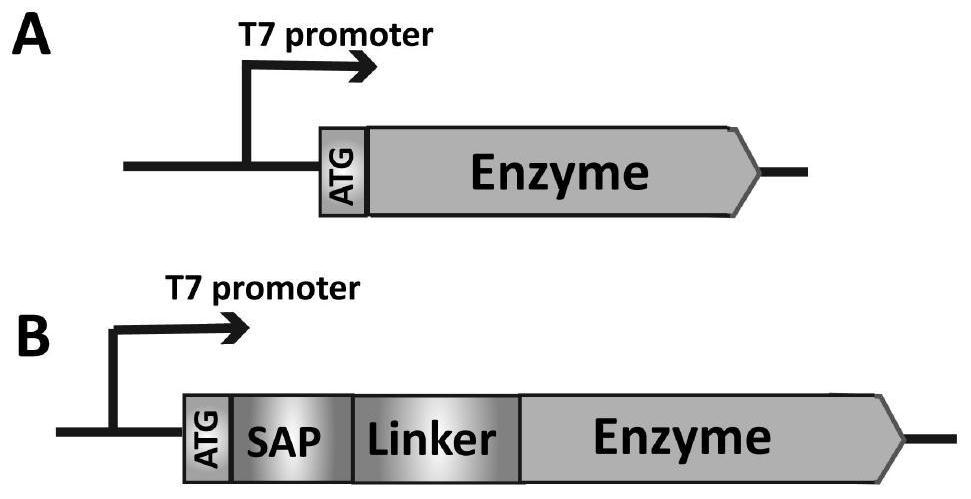
[0106] Using pET22b(+) as the plasmid backbone, the gene of lipoxygenase (LOX) was cloned between Nco I and Xho I to obtain the recombinant plasmid pET22b(+) / lox expressing wild LOX, and the coding of SAP and linker The gene was inserted between Nde I and Nco I of pET22b(+) / lox (such as figure 1 shown), the plasmids pET22b(+) / sap1-L1-lox and pET22b(+) / sap2-L2-lox expressing LOX fusion enzymes SAP1-L1-LOX and SAP2-L2-LOX were respectively obtained and transferred into Expression host Escherichia coli (Escherichia coli) BL21 (DE3) (SAP1-L1-LOX comprises the lipoxygenase of amino acid sequence as shown in SEQ ID NO.5 and the linker linking by amino acid sequence as shown in SEQ ID NO.6 The amino acid sequence at the lipoxygenase N-terminal is SAP show...
Embodiment 2
[0117] Embodiment 2: the preparation of fusion enzyme and wild enzyme
[0118] Specific steps are as follows:
[0119] Picking the single bacterium colony of the recombinant bacterium that embodiment 1 obtains is inserted in the Erlenmeyer flask (250mL) of 25mL, 37 ℃ of culture temperature, shaker speed 200r / min, cultivate 12h, obtain seed liquid; Press 3% Inoculum size Put the seed solution into a conical flask (250mL) with a liquid volume of 25mL, cultivate at a temperature of 37°C, and when the OD 600 When it reaches 0.6, add IPTG induction (wherein the induction amount of LOX is 1 mM, PGL is 0.04 mM, and ASN is 1 mM)), and at the same time adjust the temperature to the most suitable induction temperature for the enzyme and cultivate (wherein, LOX is 20 ° C for 24 h, PGL was cultured at 30°C for 48h, and ASN was cultured at 30°C for 12h), to obtain fusion enzymes containing different fusion enzymes SAP1-L1-LOX, SAP2-L2-LOX, SAP1-L1-PGL, SAP2-L2-PGL, SAP1-L1-ASN, Fermentat...
Embodiment 3
[0120] Embodiment 3: the impact of different stabilizers on fusion enzyme and wild enzyme
[0121] Specific steps are as follows:
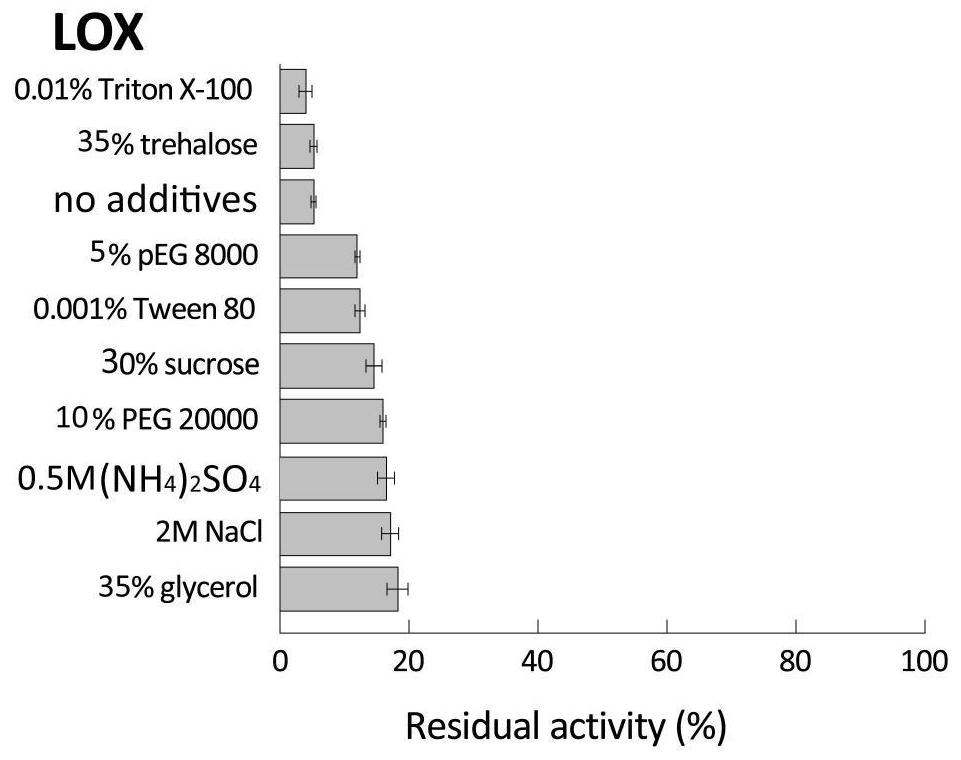
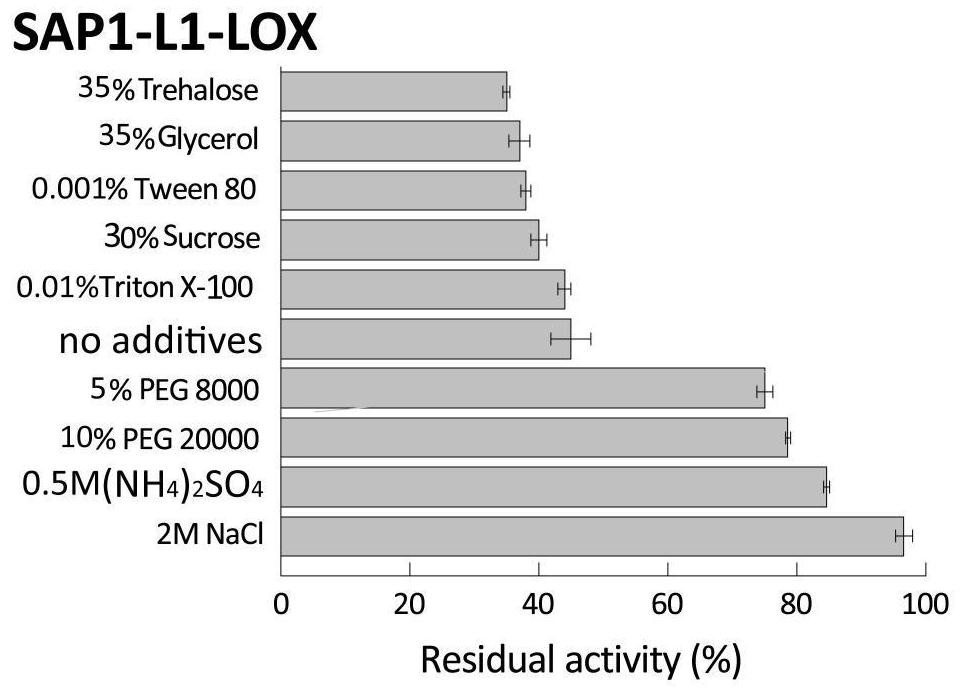
[0122] The different fusion enzymes SAP1-L1-LOX, SAP2-L2-LOX, SAP1-L1-PGL, SAP2-L2-PGL, SAP1-L1-ASN, SAP2-L2-ASN and wild enzyme LOX obtained in Example 2, The fermentation broth of PGL and ASN is purified to obtain pure enzyme liquid; with the pure enzyme liquid without adding any stabilizer as a contrast, after adding different types and concentrations of stabilizers in the pure enzyme liquid as shown in Table 1-3, the The pure enzyme solution of the control group (without stabilizer added) and the experimental group (with stabilizer added) was tested for thermal stability (the test results were as follows: Figure 2-10 ).
[0123] Table 1 Types and concentrations of stabilizers added to LOX pure enzyme solution
[0124] stabilizer concentration Polyethylene glycol octylphenyl ether (Triton X-100) 0.01% (v / v) Tween (Tw...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com