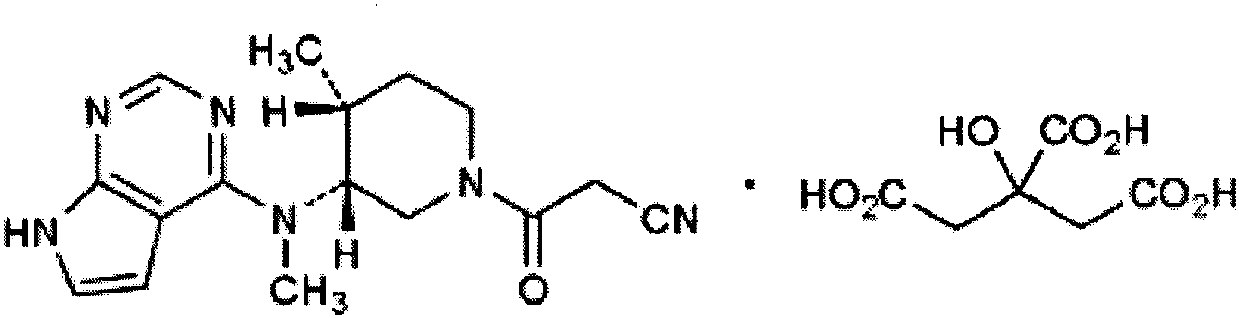
Tofacitinib citrate composition and preparation method thereof
A technology of tofacitinib and citric acid, which is applied in the field of medicine and can solve problems such as inability to administer drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
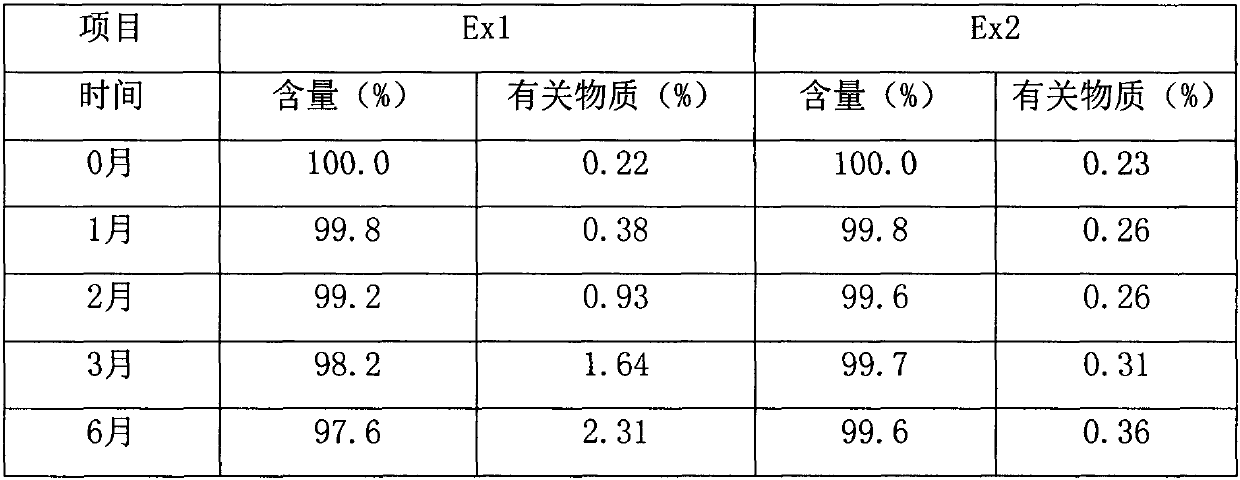
Embodiment 1
[0053] prescription:
[0054] Tofacitinib citrate (according to Tofacitinib) 50mg
[0055] Sucralose 30mg
[0056] Proper amount of citric acid (final solution pH value to 3.8)
[0057] Orange flavor 20mg
[0058] Benzyl alcohol 50mg
[0059] Water, add appropriate amount to 100ml
[0060] Preparation method: Add the prescribed amount of tofacitinib citrate, sucralose, orange essence, and benzyl alcohol into 85% purified water, stir well to dissolve it, and then adjust the pH value of the solution with 1mol / L citric acid solution 3.46, add water to the full amount, and check the pH value again to be 3.83, that is to say. This oral solution is marked as Ex2.
Embodiment 2
[0062] prescription:
[0063] Tofacitinib citrate (according to Tofacitinib) 50mg
[0064] Sucralose 30mg
[0065] Appropriate amount of citric acid (final solution pH value to 3.9)
[0066] Orange flavor 20mg
[0067] Propionic acid 50mg
[0068] Water, add appropriate amount to 100ml
[0069] Preparation method: Add the prescribed amount of tofacitinib citrate, sucralose, orange essence, and propionic acid into 85% purified water, stir well to dissolve it, and then adjust the pH value of the solution with 1mol / L citric acid solution 3.38, make up the water to the full amount, and check the pH value again to be 3.89, that is to say. This oral solution is marked as Ex2.
Embodiment 3
[0071] prescription:
[0072] Tofacitinib citrate (according to Tofacitinib) 10mg
[0073] Sucrose 10g
[0074] Appropriate amount of hydrochloric acid (final solution pH value to 3.3)
[0075] Strawberry flavor 5mg
[0076] Sodium Benzoate 25mg
[0077] Water, add appropriate amount to 100ml
[0078] Preparation method: Add the prescribed amount of tofacitinib citrate, strawberry essence, and sodium benzoate into 85% purified water, stir well to dissolve, then adjust the pH value of the solution to 3.04 with 1mol / L hydrochloric acid solution, and make up the water to the full amount , and the pH value was detected again to be 3.32, that is to say. The oral solution is labeled Ex3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com