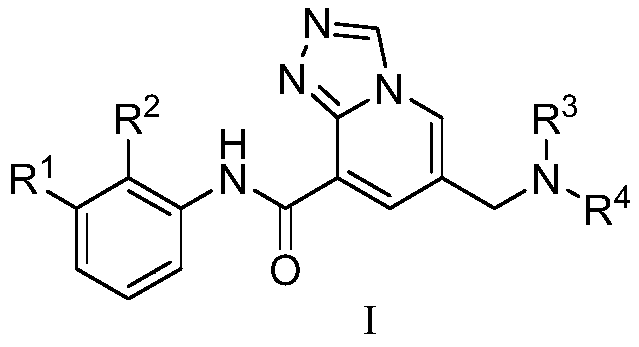
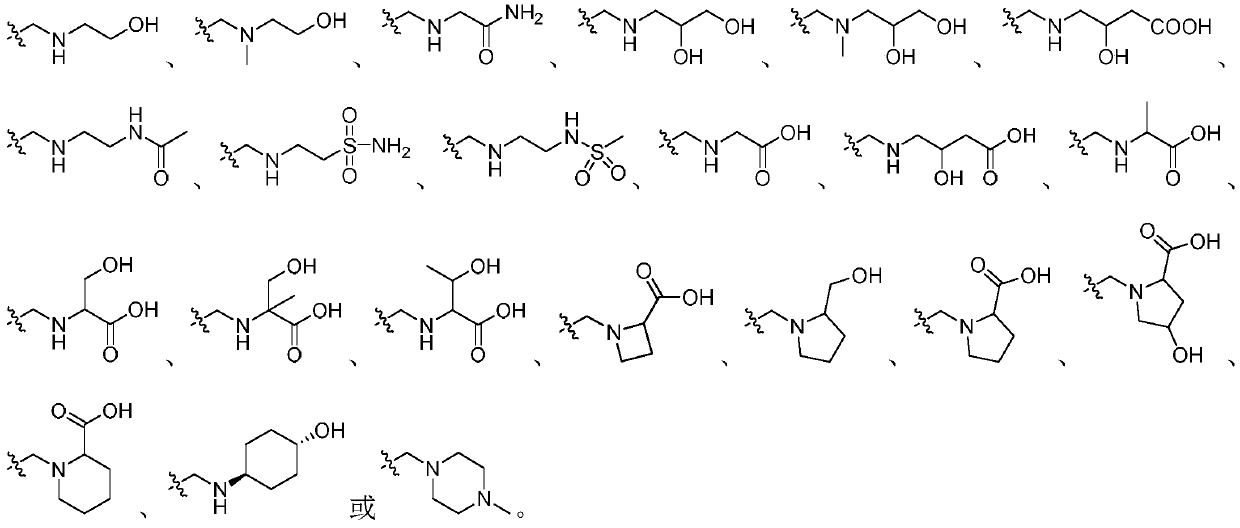
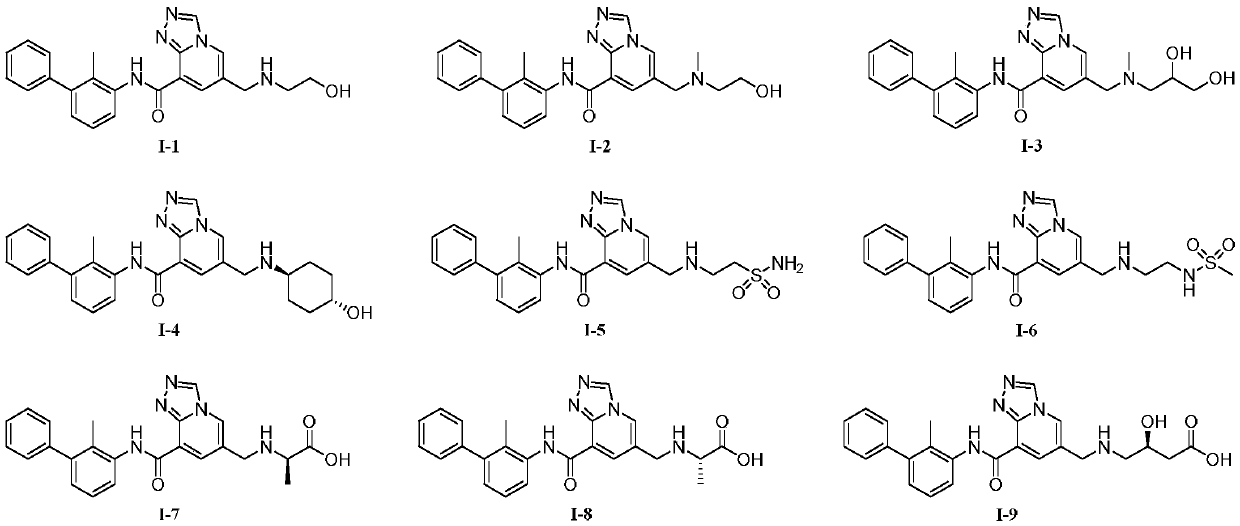
Triazolopyridine compound and preparation method and application thereof
A technology of azolopyridine and compound, applied in the direction of digestive system, organic chemistry, drug combination, etc., can solve the problems of immunogenic side effects, decomposition, and only injection administration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Example 1: 6-(((2-hydroxyethyl)amino)methyl)-N-(2-methyl-[1,1'-biphenyl]-3-yl)-[1,2,4 ]Triazolo[4,3-a]pyridine-8-carboxamide (I-1)
[0064]
[0065] Step 1: 2-Methyl-[1,1'-biphenyl]-3-amine
[0066]
[0067] At room temperature, 3-bromo-2-methylaniline (15g, 0.081mol), phenylboronic acid (12.2g, 0.1mol), palladium acetate (1.82g, 8.1mmol), potassium carbonate (24.9g, 0.18mol) were added into a mixed solution of ethanol / water (volume ratio 1:1, 100mL), in N 2 The reaction was stirred for 5 hours under protection. After completion of the reaction, filter with suction, evaporate the filtrate to dryness, and separate by column chromatography to obtain 12.6 g of a light yellow solid with a yield of 85.2%.
[0068] Step 2: Methyl 5-bromo-2-hydrazinonicotinate
[0069]
[0070] At room temperature, dissolve methyl 5-bromo-2-chloronicotinate (13.55g, 0.054mol) in 1,4-dioxane (150mL), add hydrazine hydrate (6g, 0.096mol), at 60°C React for 3 hours. After the react...
Embodiment 2
[0091] Example 2: 6-(((2-hydroxyethyl)(methyl)amino)methyl)-N-(2-methyl-[1,1'-biphenyl]-3-yl)-[1 ,2,4]triazolo[4,3-a]pyridine-8-carboxamide (I-2)
[0092]
[0093] ESI-MS m / z:416.2[M+H] + ; 1 H NMR (600MHz, DMSO-d 6 )δ11.40(s,1H),9.19(s,1H),8.75(s,1H),8.48(d,J=1.3Hz,1H),8.19(d,J=7.9Hz,1H),7.48( t,J=7.5Hz,2H),7.42–7.33(m,4H),7.09(d,J=7.3Hz,1H),4.51(t,J=5.4Hz,1H),3.72(s,2H), 3.56(q, J=6.0Hz, 2H), 2.54(t, J=6.1Hz, 2H), 2.35(s, 3H), 2.24(s, 3H).
Embodiment 3
[0094] Example 3: 6-(((2,3-dihydroxypropyl)(methyl)amino)methyl)-N-(2-methyl-[1,1'-biphenyl]-3-yl) -[1,2,4]triazolo[4,3-a]pyridine-8-carboxamide (I-3)
[0095]
[0096] ESI-MS m / z:446.2[M+H] + ; 1 H NMR (600MHz, DMSO-d 6 )δ11.40(s,1H),9.20(s,1H),8.75(s,1H),8.48(s,1H),8.20(d,J=8.0Hz,1H),7.48(t,J=7.5 Hz,2H),7.40(t,J=7.4Hz,1H),7.38–7.36(m,2H),7.34(d,J=7.9Hz,1H),7.09(d,J=7.5Hz,1H), 4.54(br,2H),3.74(s,2H),3.69(dd,J=9.9,5.0Hz,1H),3.38(d,J=5.3Hz,1H),3.32(d,J=5.8Hz,1H ),2.54(dd,J=12.8,4.6Hz,1H),2.40(dd,J=12.7,7.1Hz,1H),2.35(s,3H),2.25(s,3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com