Quality evaluation method for Chinese herbal compound aconite lizhong decoction for treating gastric ulcer
A technology for the management and quality evaluation of aconite, which is applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve the problems of failing to achieve quality control, and cannot accurately reflect the compound prescription of traditional Chinese medicine, etc., and achieve high sensitivity, simple and easy determination method, The effect of rapid evaluation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
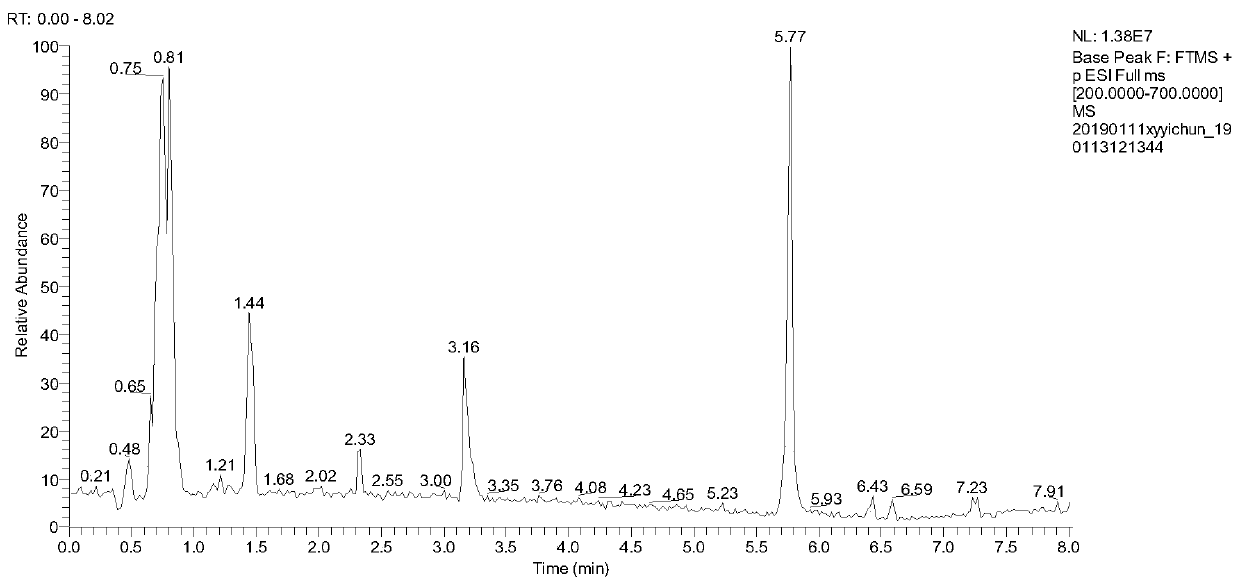
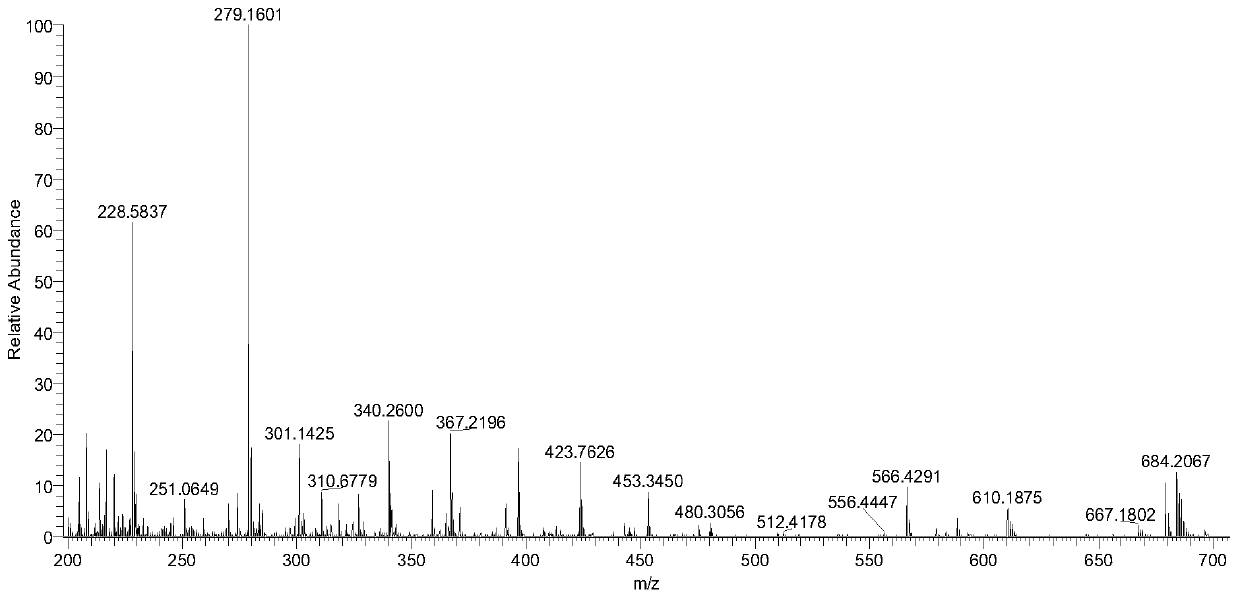
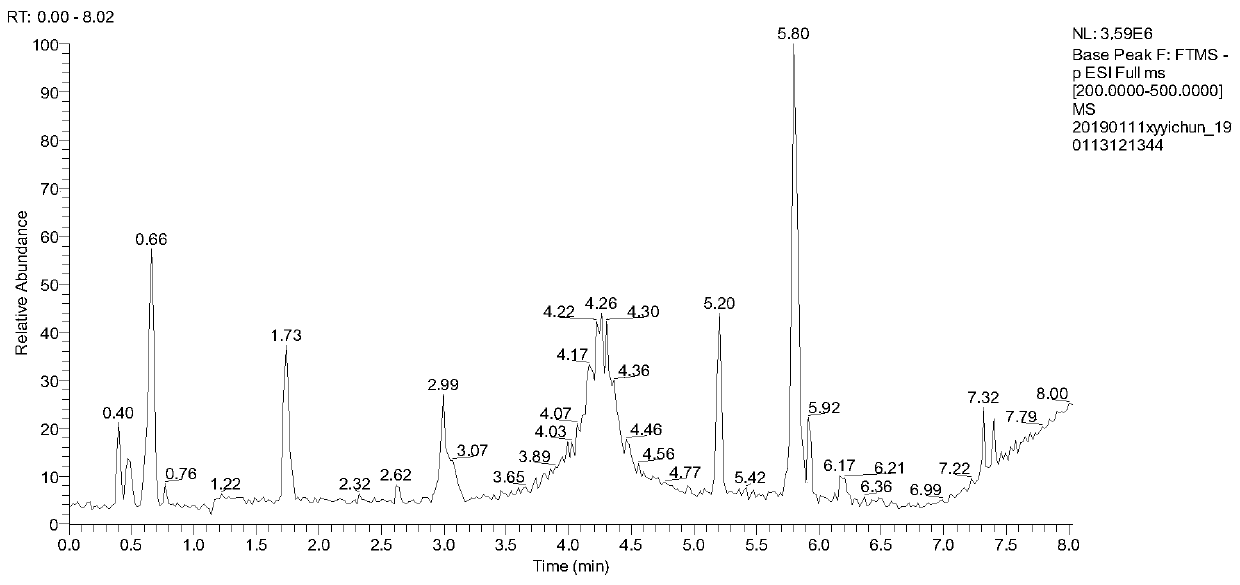
[0038] According to the quality evaluation method of the traditional Chinese medicine Fuzi Lizhong Decoction for the treatment of gastric ulcer in this embodiment, the content limits of the 10 chemical components in the traditional Chinese medicine Fuzi Lizhong Decoction are respectively: benzoylaconitine 2.248 μg / g~ 2.864μg / g, Benzoyl neoaconitine 16.464μg / g~17.655μg / g, Atractylodes lactone I0.632μg / g~0.743μg / g, Atractylodeslide II4.158μg / g~4.667μg / g , Atractylodes lactone III 3.788μg / g~4.043μg / g, tangshenoside 51.802μg / g~53.089μg / g, liquiritin 15.664μg / g~16.987μg / g, liquiritin 5.918μg / g~6.042μg / g, 8-gingerol 2.282μg / g~3.127μg / g and 10-gingerol 1.600μg / g~1.985μg / g are qualified.
[0039] The composition of the traditional Chinese medicine compound aconite Lizhong Decoction is prepared aconite, codonopsis, dried ginger, atractylodes macrocephala and licorice of equal quality.
[0040]This embodiment also provides a quality evaluation method for the above-mentioned Chinese me...
Embodiment 2
[0058] According to the quality evaluation method of the traditional Chinese medicine Fuzi Lizhong Decoction for the treatment of gastric ulcer in this embodiment, the content limits of the 10 chemical components in the traditional Chinese medicine Fuzi Lizhong Decoction are respectively: benzoylaconitine 2.248 μg / g~ 2.864μg / g, Benzoyl neoaconitine 16.464μg / g~17.655μg / g, Atractylodes lactone I0.632μg / g~0.743μg / g, Atractylodeslide II4.158μg / g~4.667μg / g , Atractylodes lactone III 3.788μg / g~4.043μg / g, tangshenoside 51.802μg / g~53.089μg / g, liquiritin 15.664μg / g~16.987μg / g, liquiritin 5.918μg / g~6.042μg / g, 8-gingerol 2.282μg / g~3.127μg / g and 10-gingerol 1.600μg / g~1.985μg / g are qualified.
[0059] The composition of the traditional Chinese medicine compound aconite Lizhong Decoction is prepared aconite, codonopsis, dried ginger, atractylodes macrocephala and licorice of equal quality.
[0060] This embodiment also provides a quality evaluation method for the above-mentioned Chinese m...
Embodiment 3
[0075]According to the quality evaluation method of the traditional Chinese medicine Fuzi Lizhong Decoction for the treatment of gastric ulcer in this embodiment, the content limits of the 10 chemical components in the traditional Chinese medicine Fuzi Lizhong Decoction are respectively: benzoylaconitine 2.248 μg / g~ 2.864μg / g, Benzoyl neoaconitine 16.464μg / g~17.655μg / g, Atractylodes lactone I0.632μg / g~0.743μg / g, Atractylodeslide II4.158μg / g~4.667μg / g , Atractylodes lactone III 3.788μg / g~4.043μg / g, tangshenoside 51.802μg / g~53.089μg / g, liquiritin 15.664μg / g~16.987μg / g, liquiritin 5.918μg / g~6.042μg / g, 8-gingerol 2.282μg / g~3.127μg / g and 10-gingerol 1.600μg / g~1.985μg / g are qualified.
[0076] The composition of the traditional Chinese medicine compound aconite Lizhong Decoction is prepared aconite, codonopsis, dried ginger, atractylodes macrocephala and licorice of equal quality.
[0077] This embodiment also provides a quality evaluation method for the above-mentioned Chinese me...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com