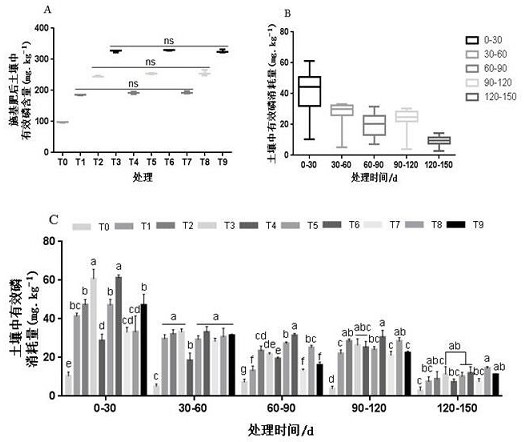
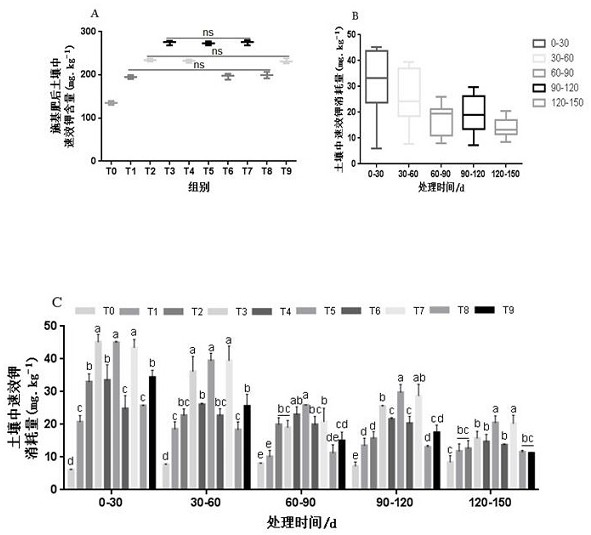
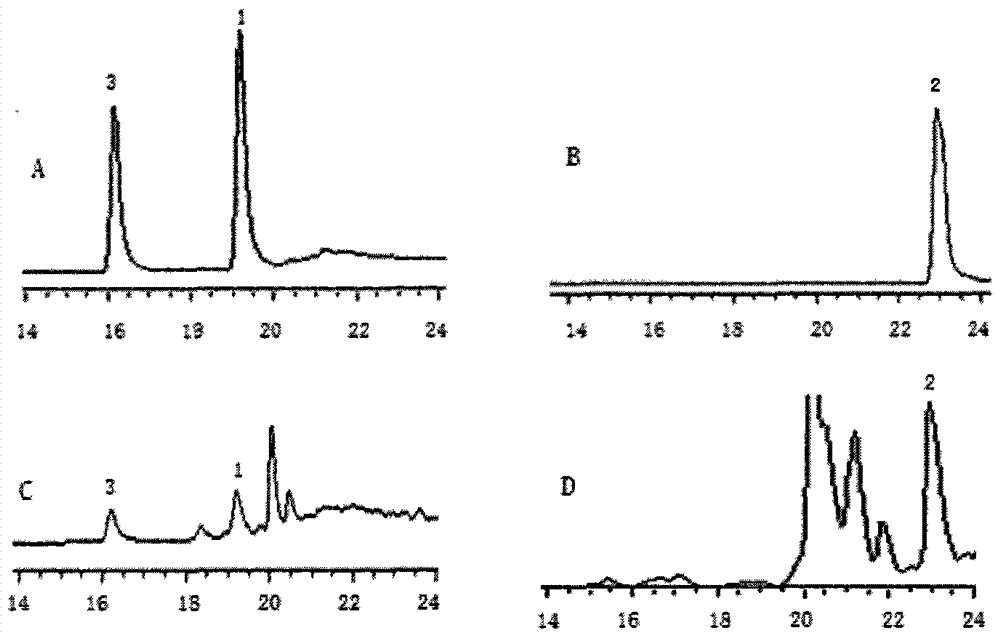
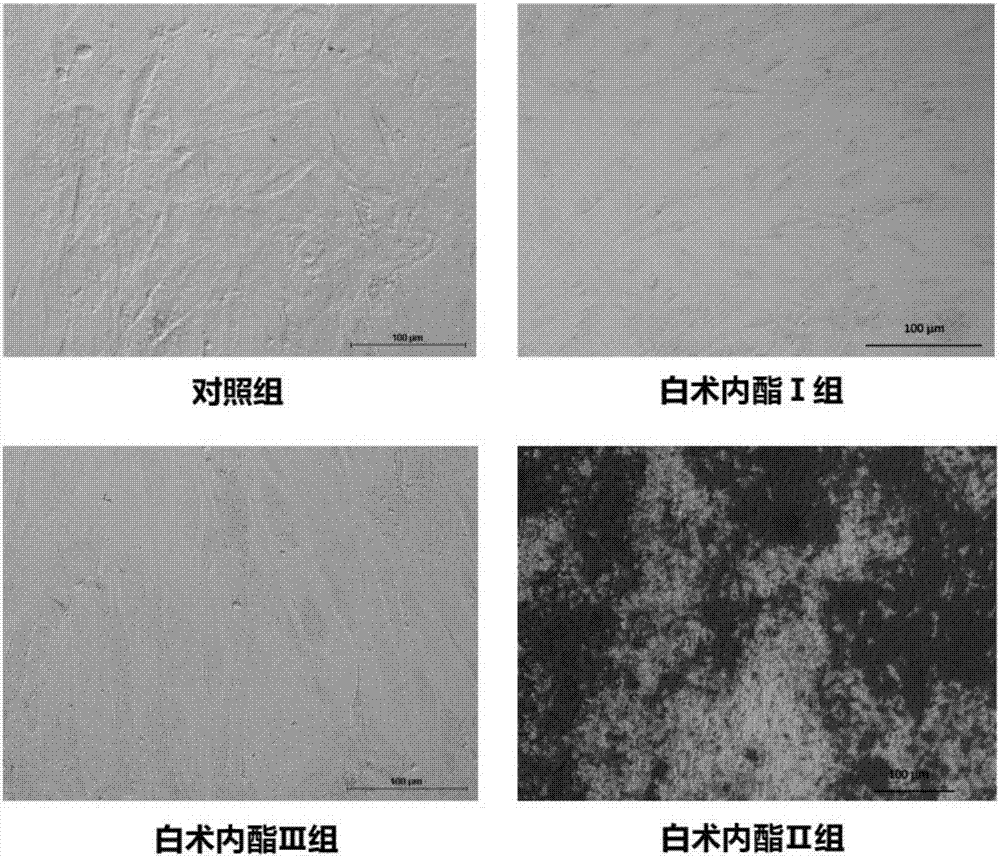
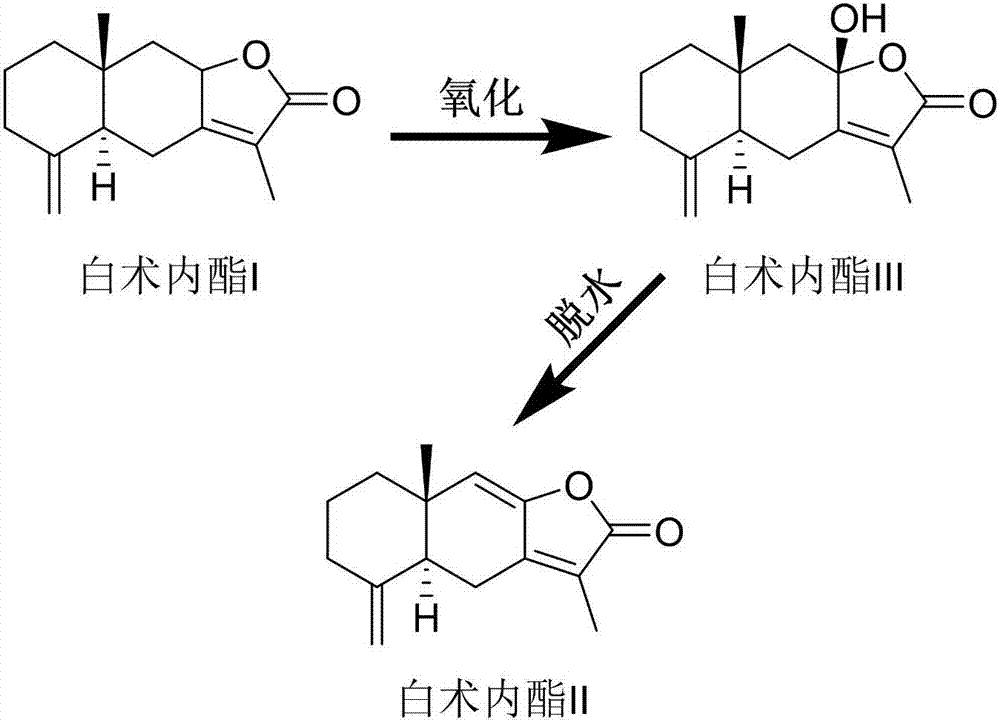
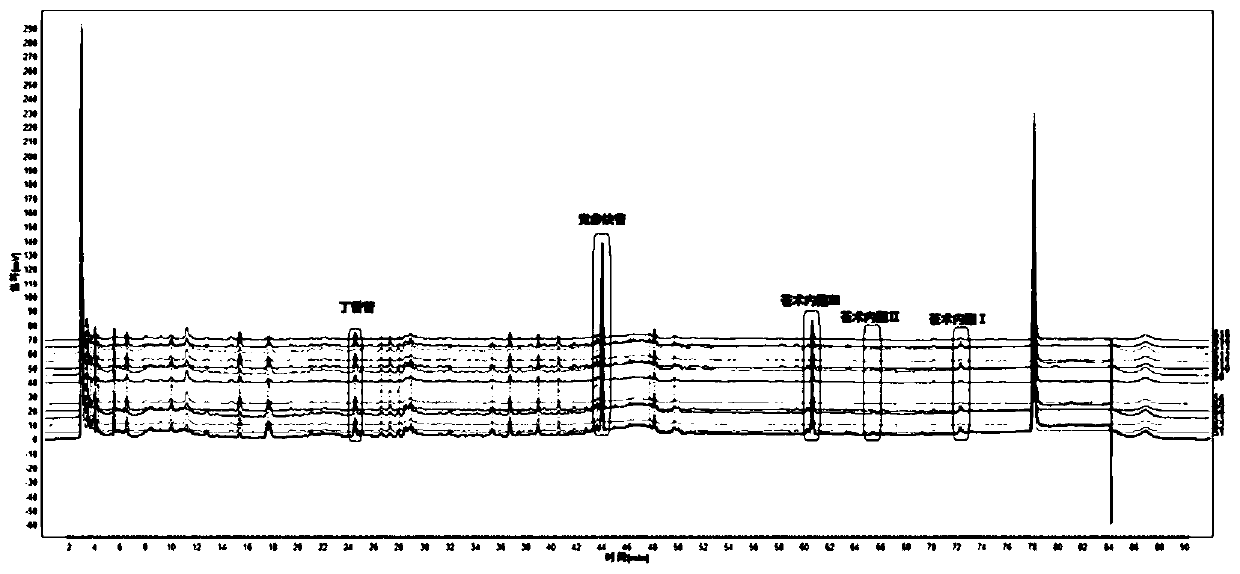
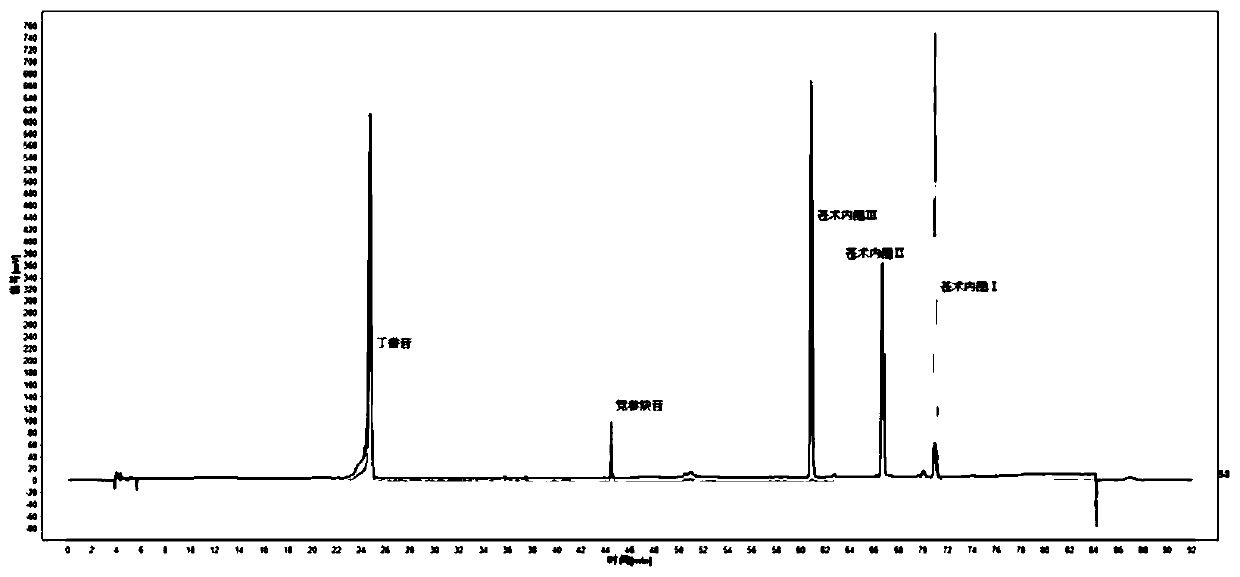
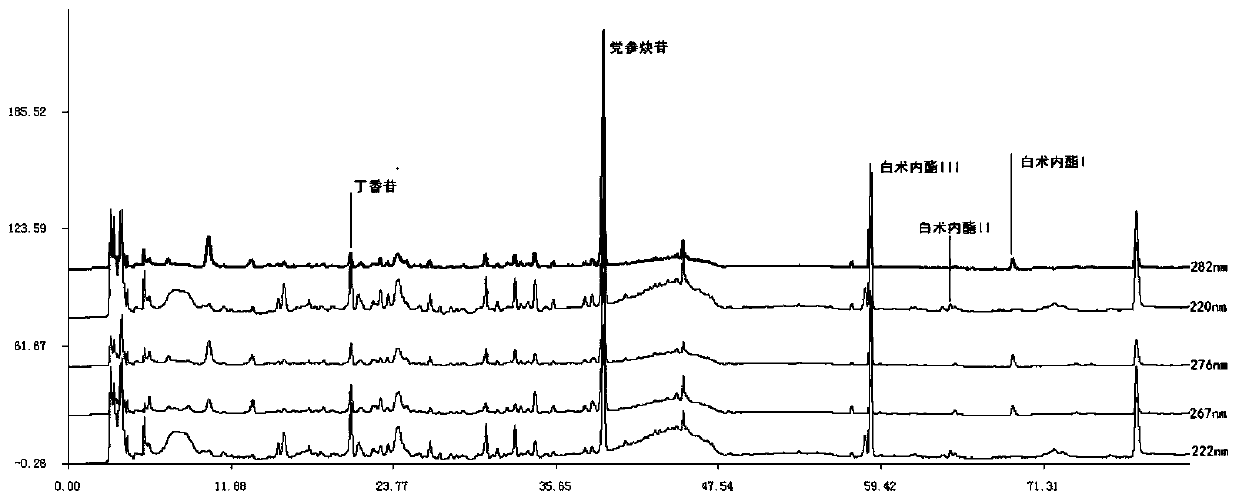
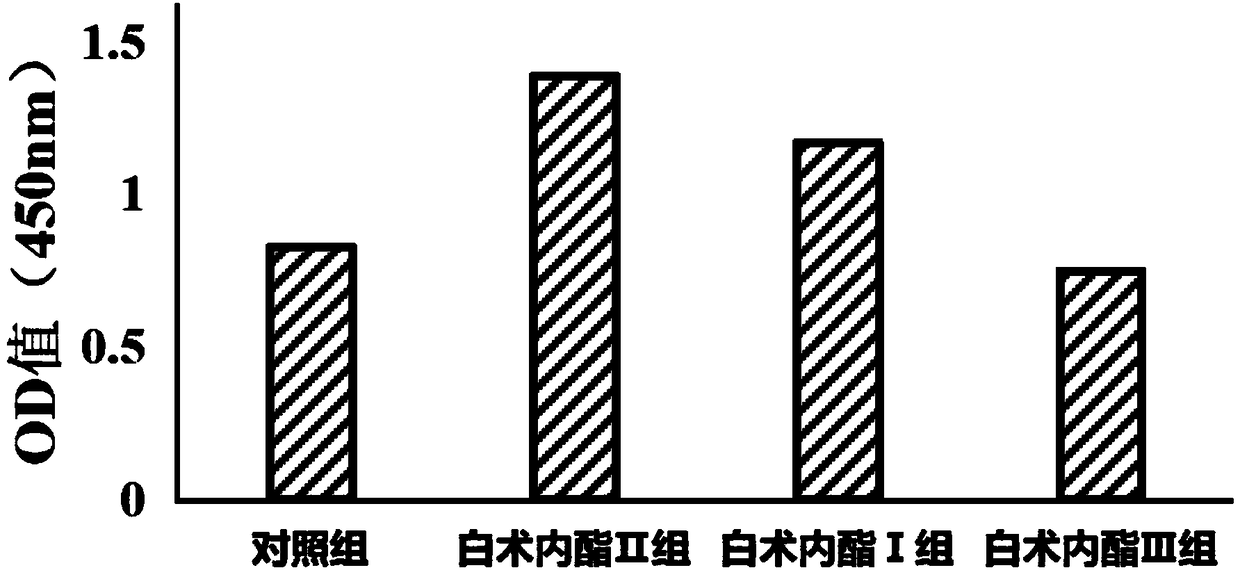
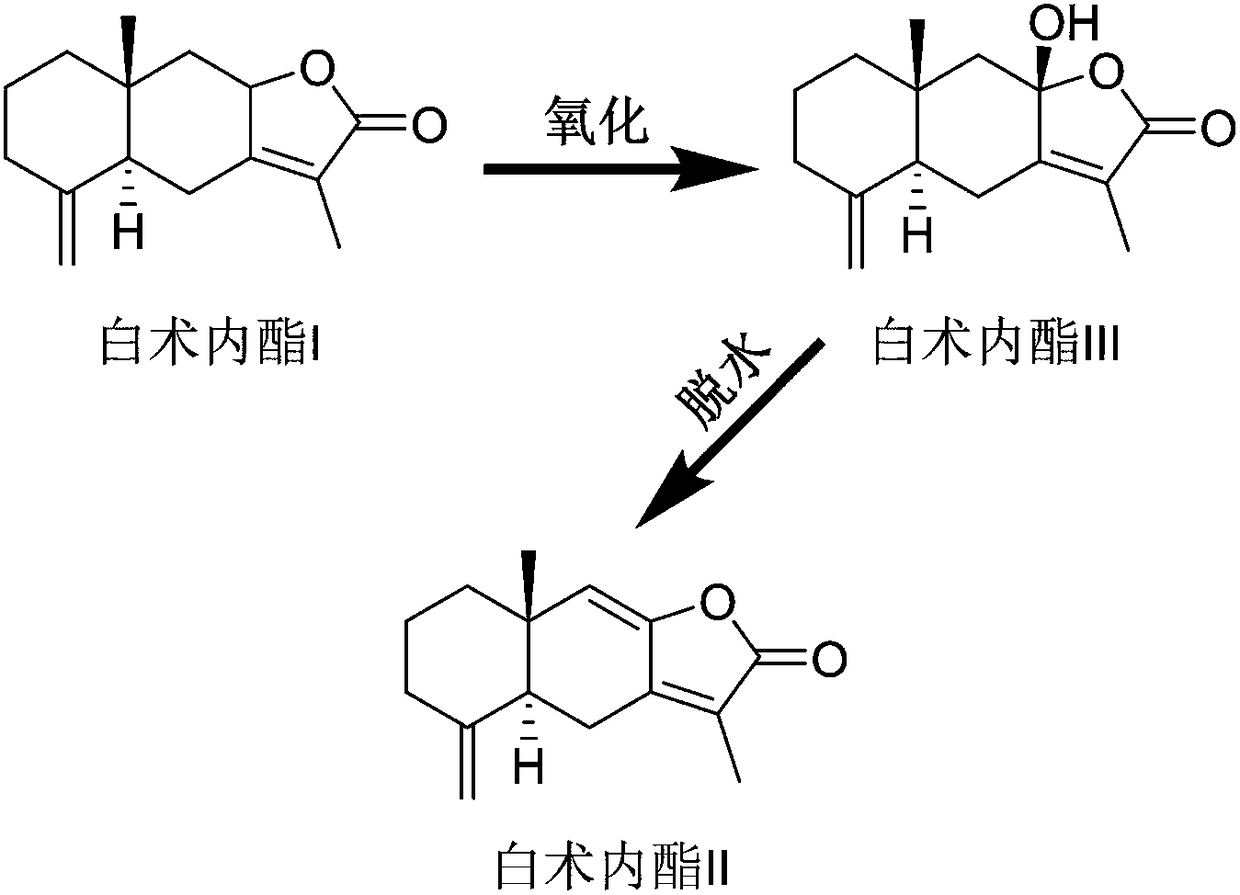
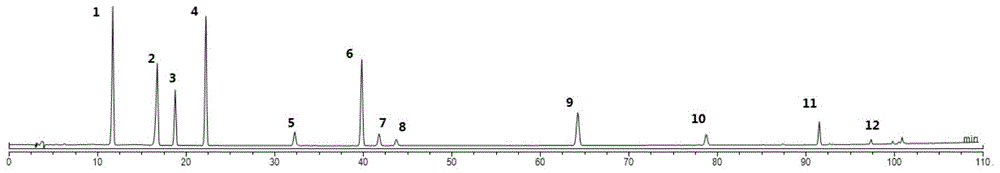
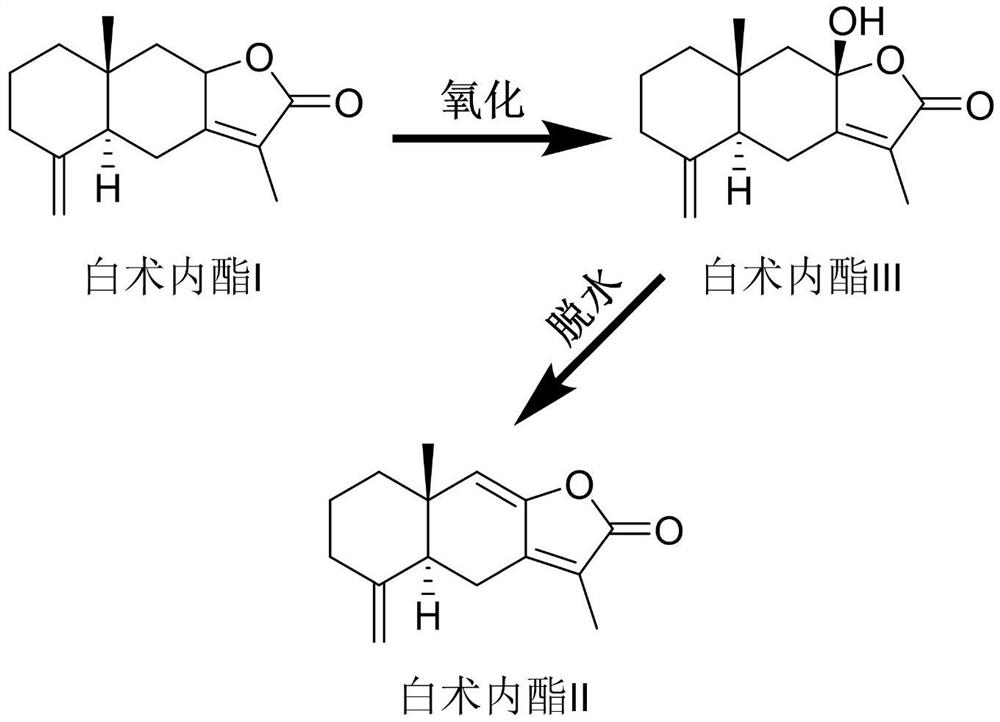
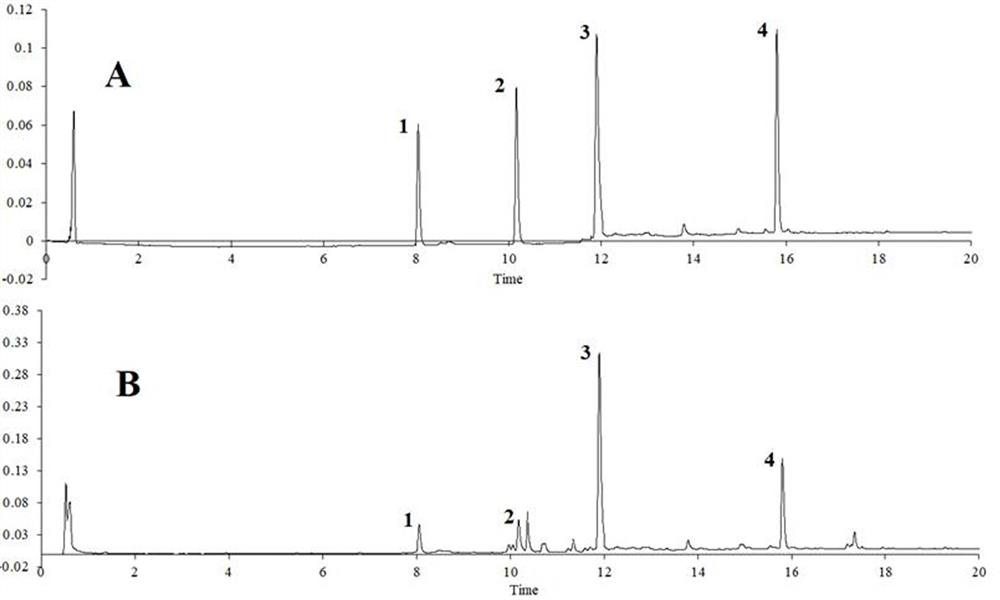
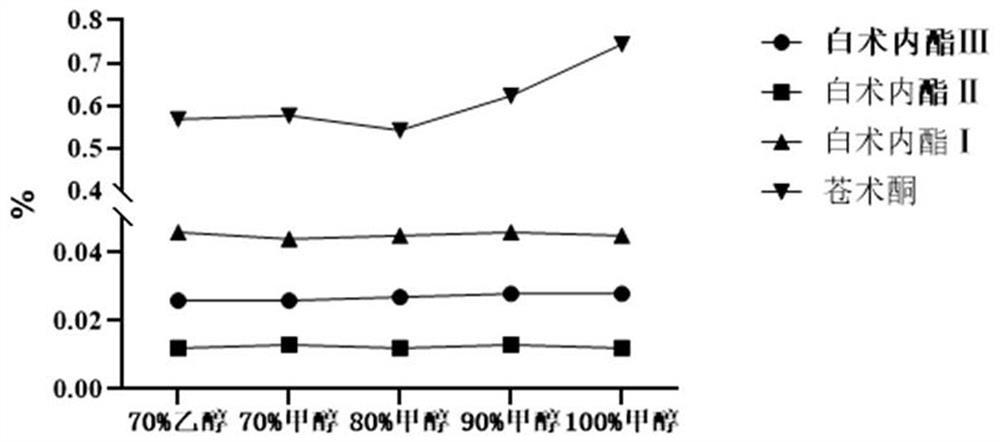
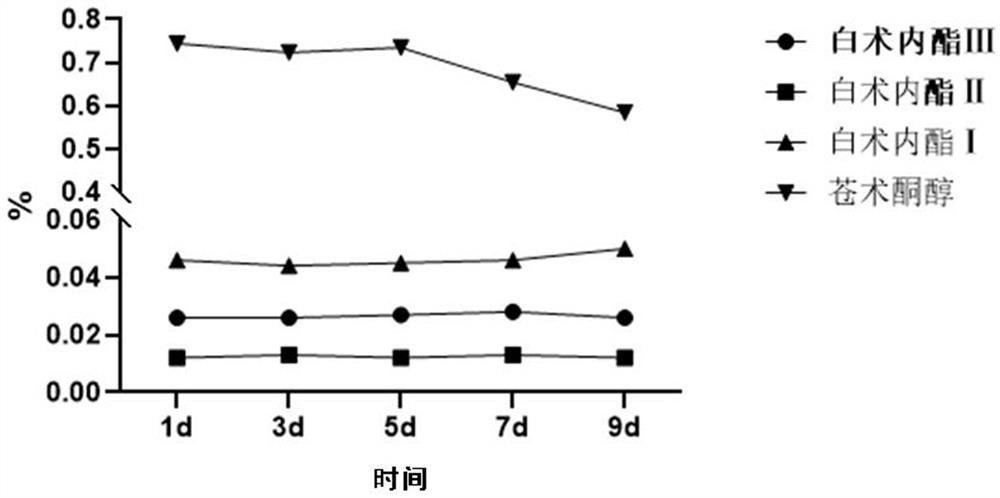
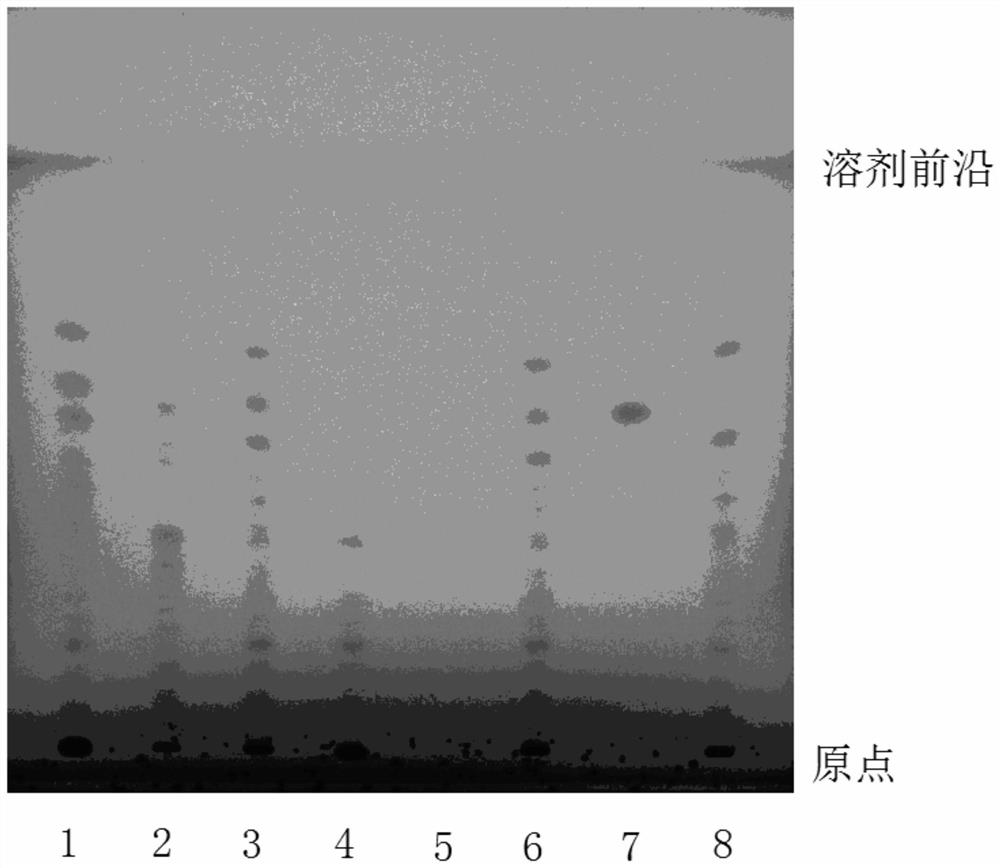
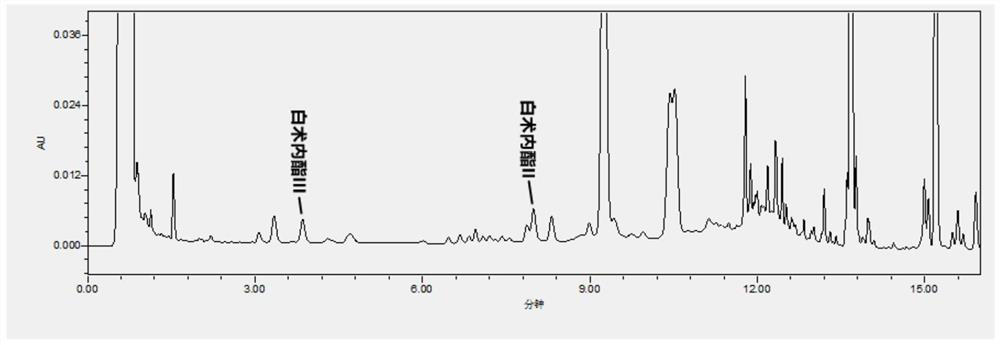
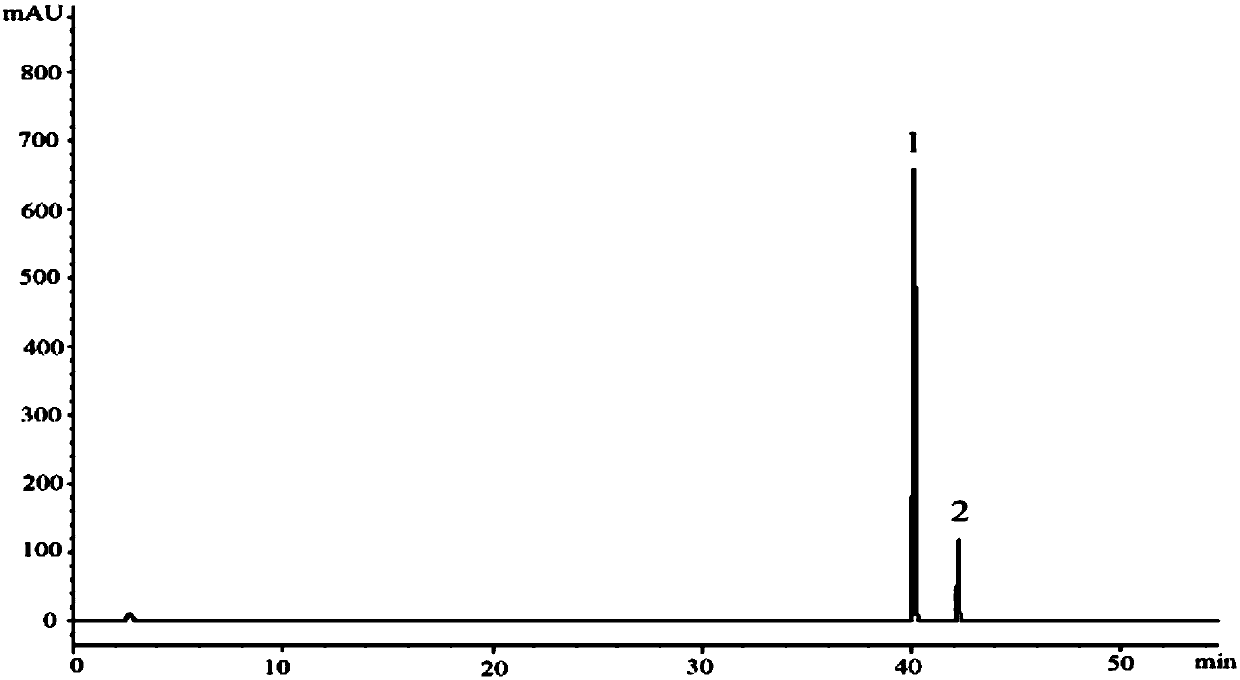
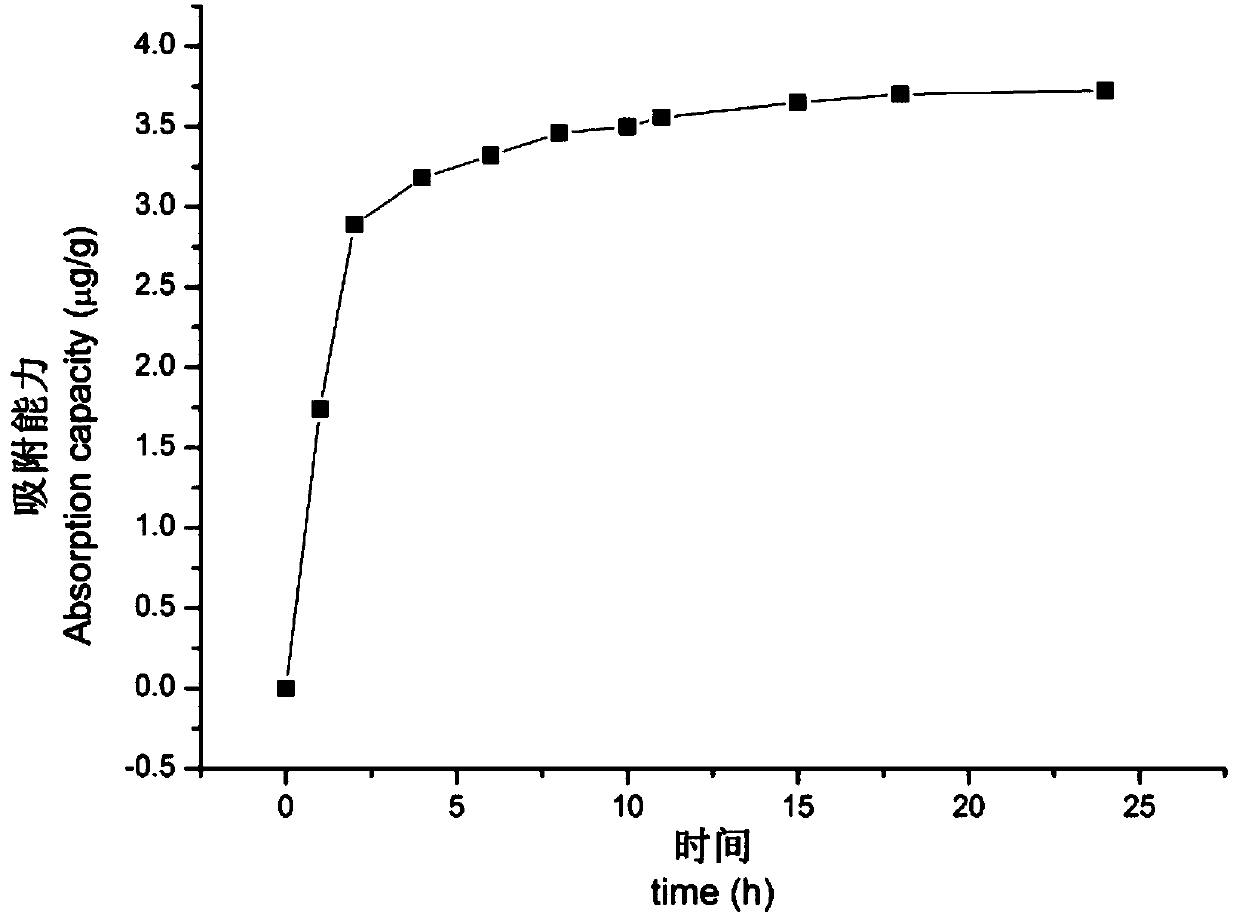
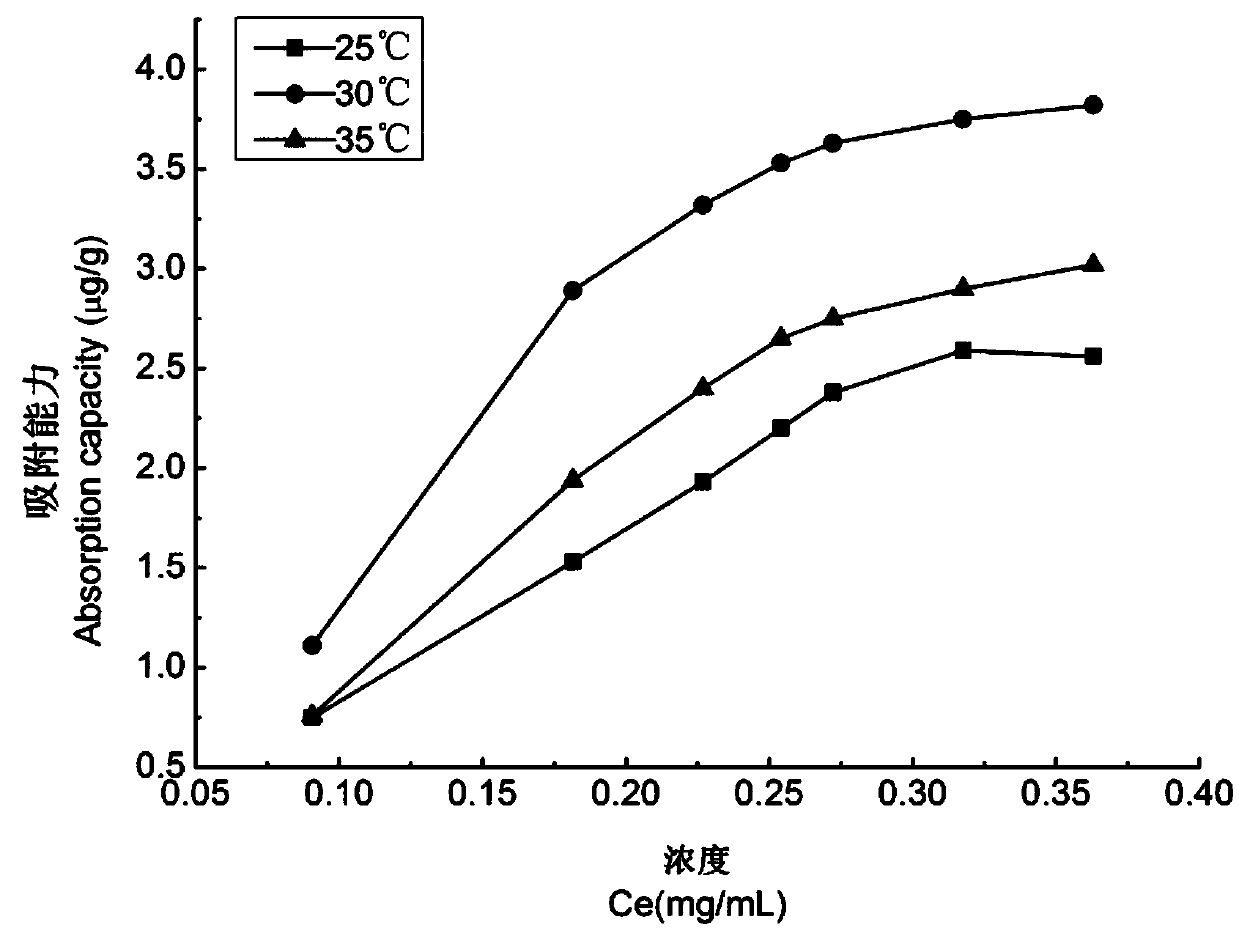
Patents
Literature
34 results about "Atractylenolide II" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Method for separating effective constituent butenolide II from astraolylis lancea formalyrata volatile oil
InactiveCN101265247AIncrease contentEasy to operateOrganic chemistryPlant ingredientsChromatographic separationAtractylenolide II
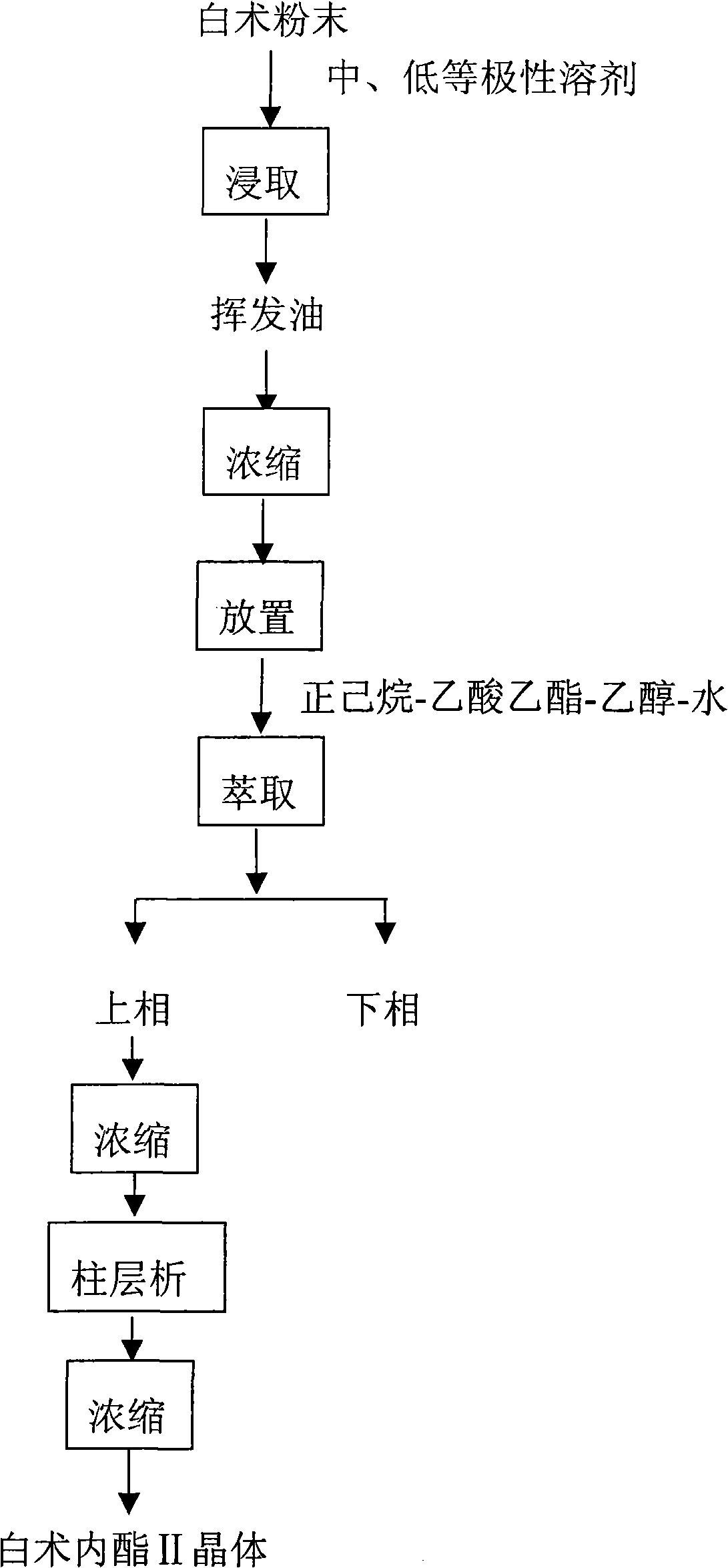
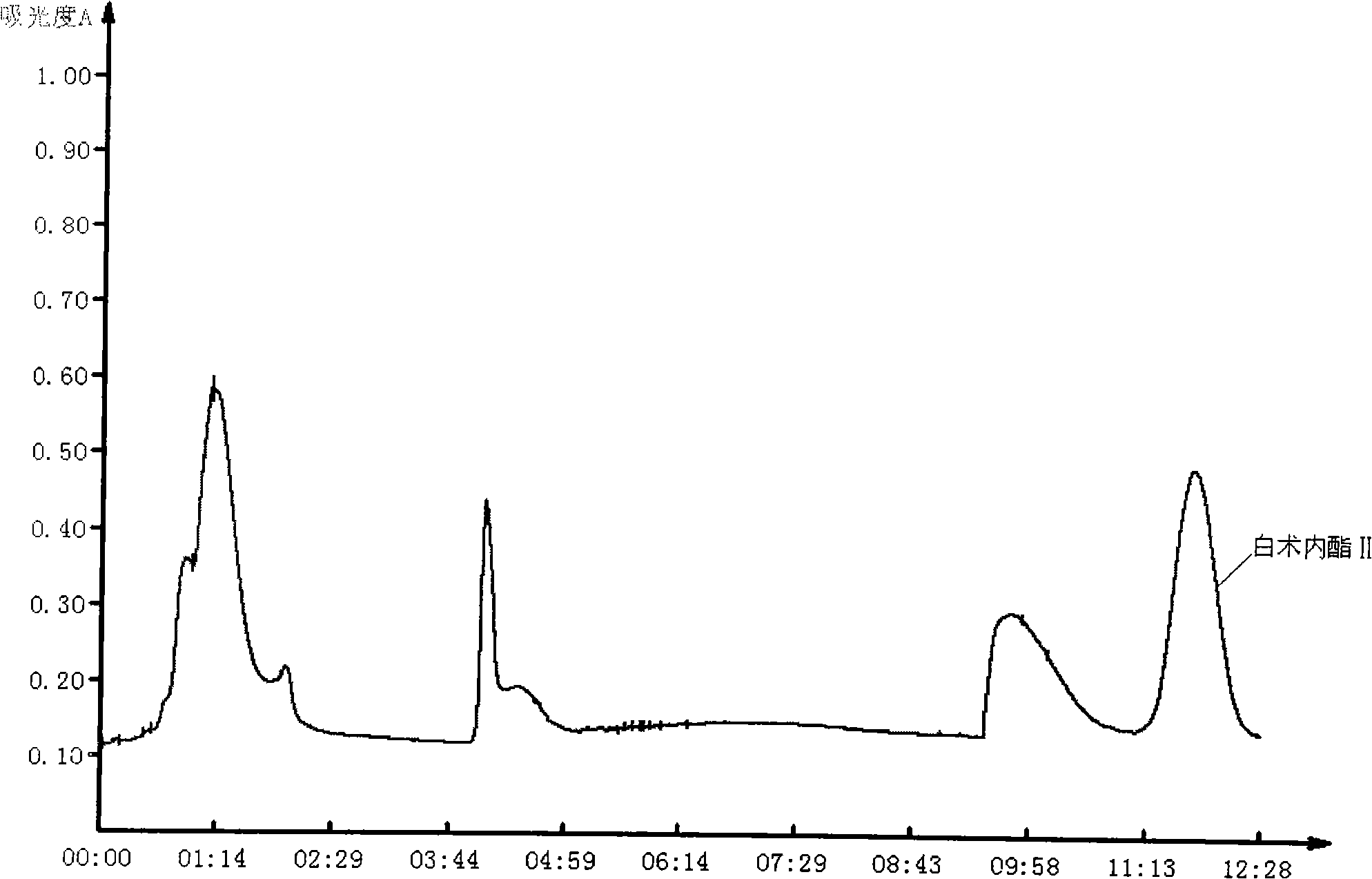
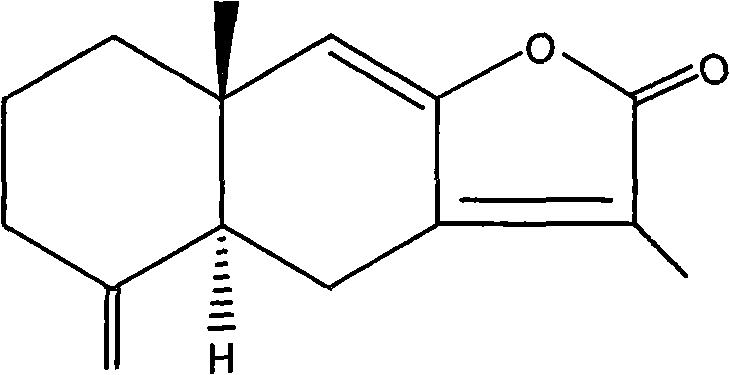
The invention discloses a method for separating effective component, atractylenolide II, from Rhizoma Atractylodis Macrocephalae volatile oil. The method includes pulverizing rhizome of dried Rhizoma Atractylodis Macrocephalae, extracting in medium and low polar solvents, and concentrating to obtain tan volatile oil; standing for a certain time, extracting the volatile oil with mixed solution prepared from n-hexane, ethyl acetate, ethanol and water at a certain ratio, collecting upper layer phase, and concentrating to obtain the refined volatile oil; and gradient eluting with mixed solvent of petroleum ether and ethyl acetate at a certain ratio with column chromatography, collecting correspondent fraction, concentrating, and recycling solvent to obtain atractylenolide II. The method performs pretreatment on Rhizoma Atractylodis Macrocephalae volatile oil coarse product by extracting and oxidizing before separating through column chromatography to have simple process and low cost, increased yield and purity of atractylenolide II, and avoids the disadvantages in the conventional post-treatment steps such as crystal loss caused by recrystallization, etc.
Owner:ZHEJIANG UNIV
Atractylenolide extract having antiparkinsonian effect, preparation method and application thereof
InactiveCN105267274AIncrease contentHigh extraction rateNervous disorderPlant ingredientsAntiparkinsonian drugAtractylenolide III
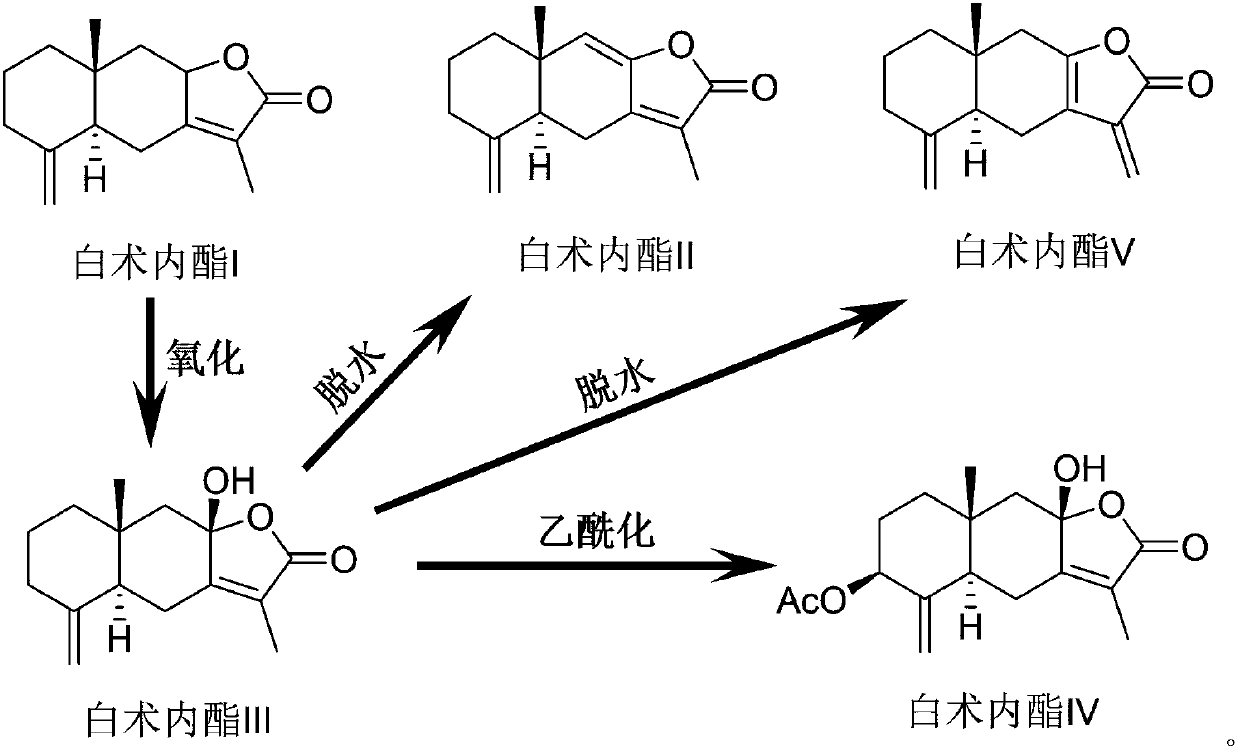
The invention discloses atractylenolide extract having antiparkinsonian effect, a preparation method and application thereof. Weight percentages of atractylenolide I, atractylenolide II and atractylenolide III in the atractylenolide extract are larger than 5%, 10% and 20% respectively, and purity of total lactone is larger than 35%. The application refers to that the atractylenolide extract is used to prepare antiparkinsonian drug. The preparation method includes that oxidant is added into white atractylode rhizome powder, and atractylone in white atractylode rhizome volatile oil is oxidized into atractylenolide compounds, so that content of atractylenolide is increased; zinc particles are added into an extraction solution to protect generated compounds such as atractylenolide from being further oxidized and degraded. The preparation method is simple, convenient and practical, easy to operate and more suitable for industrial production, and has remarkable progressiveness and practical value.
Owner:LIAONING UNIV OF TRADITIONAL CHINESE MEDICINE
Preparation method of atractylenolide II
InactiveCN103254165ASimple and fast operationSolve problemsOrganic chemistryUltraviolet detectorsAtractylenolide II
The invention discloses a preparation method of atractylenolide II, which is simple in operation and small in pollution. The method comprises the following steps: taking and crushing a bighead atractylodes rhizome raw material, adding a 70-100% methanol solution with an addition amount 5-10 times as large as the amount of the raw material into the raw material, and carrying out ultrasonic extraction on the obtained mixture 2-3 times; after an extracting solution is concentrated, carrying out extraction on the obtained product by using an ethyl acetate solution, collecting extract liquor and recovering a reagent, separating a concentrated solution again by using a high-speed countercurrent chromatograph, carrying out on-line monitoring on the obtained object by using an ultraviolet detector, and carrying out fraction collection and decompressed drying on the obtained product. In the preparation of atractylenolide II implemented by using the method, products are high in purity, and easy to realize industrialization amplification.
Owner:NANJING ZELANG AGRI DEV
Content determination method for multiple components in Yupingfeng preparation
ActiveCN105021751AStrong specificityNo interference peakComponent separationFiltrationAtractylenolide II
The invention relates to a content determination method for multiple components in a Yupingfeng preparation. The method comprises the steps of: (1) taking the Yupingfeng preparation, crushing or not crushing it, then performing precise weighing, adding methanol to conduct reflux extraction for 1-3h, carrying out filtration and concentration, then adding methanol to a constant volume, thus obtaining a test solution; (2) precisely weighing the reference substance prim-o-glucosylcimifugin, calycosin-7-glucoside, cimifugin, 5-O-methylvisammioside, sec-o-glucosylhamaudol, calycosin, formononetin, atractylenolide III, atractylenolide I and atractylenolide II respectively, and adding methanol to perform dissolving to a constant volume, thus obtaining a mixed reference solution; (3) conducting determination: precisely sucking the test solution and the mixed reference solution respectively, injecting them into a high performance liquid chromatograph, carrying out gradient elution under certain mobile phase condition, and performing multi-wavelength simultaneous monitoring, thus obtaining the content. The method provided by the invention can accurately determine the content of 10 components in the Yupingeng preparation, and can objectively, comprehensively and sensitively reflect the quality condition of the Yupingfeng preparation.
Owner:GUANGZHOU XIANGXUE PHARMA CO LTD
Application of atractylenolide-II derivative in preparation of platelet aggregation resisting drug and platelet aggregation resisting drug
ActiveCN104490868ASimple compositionGood curative effectOrganic active ingredientsAntipyreticTreatment effectSide effect
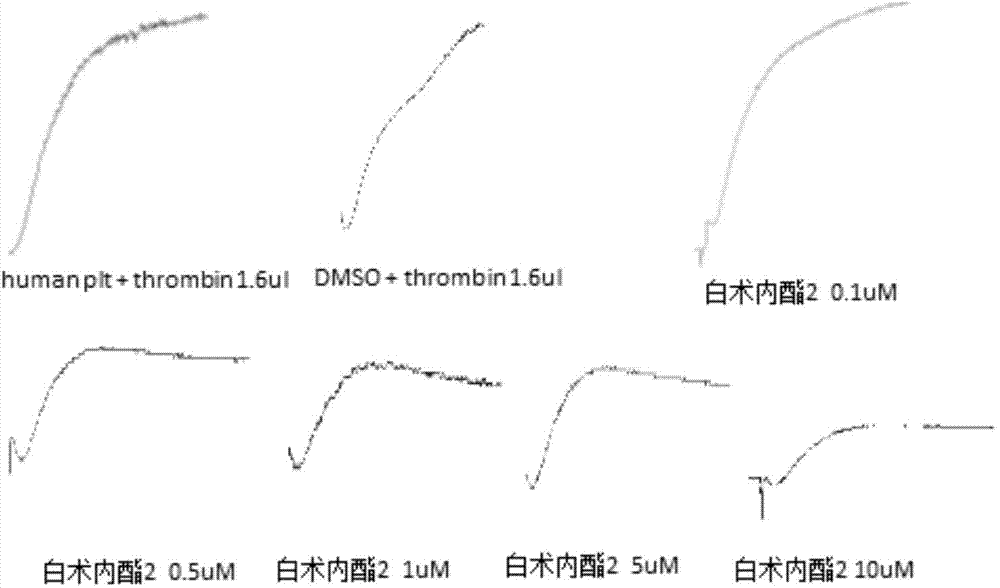
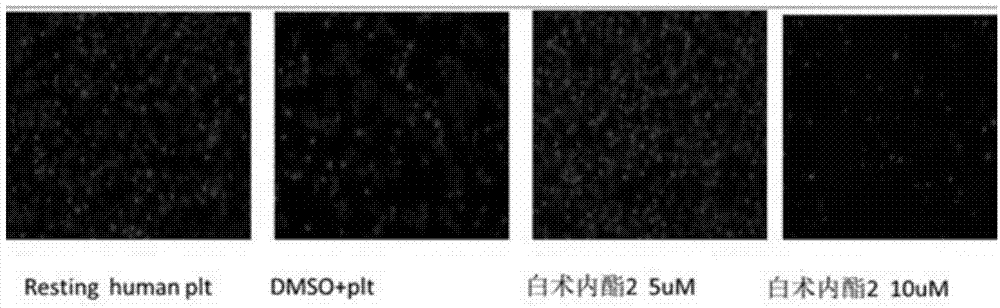
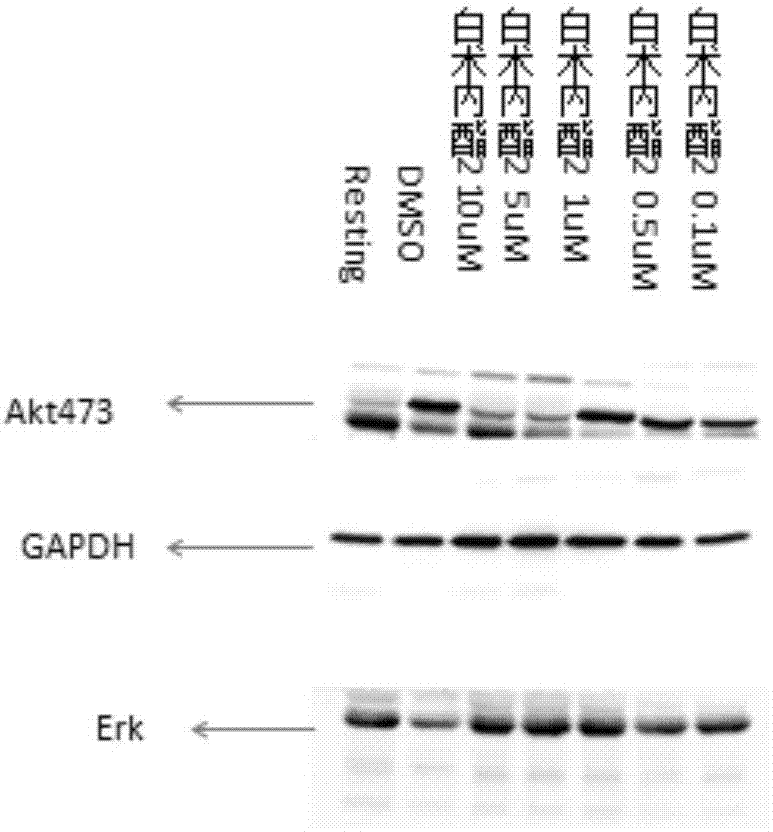
The invention relates to a platelet aggregation resisting drug. The platelet aggregation resisting drug comprises an atractylenolide-II derivative represented by a structural formula 1 shown in specifications. The invention further relates to application of an atractylenolide-II derivative in preparation of the platelet aggregation resisting drug. The platelet aggregation resisting drug, which is simple in composition and is prepared from effective ingredients of natural medicinal raw materials or effective ingredient extracts of the medicinal raw materials, provided by the invention has a good treatment effect, is free from toxic or side effects, is not prone to the generation of tolerance, is convenient to take and is generally applicable to the problems, such as viscous blood and thrombus, caused by too-high platelet aggregation rate.
Owner:SHANGHAI NINTH PEOPLES HOSPITAL AFFILIATED TO SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE
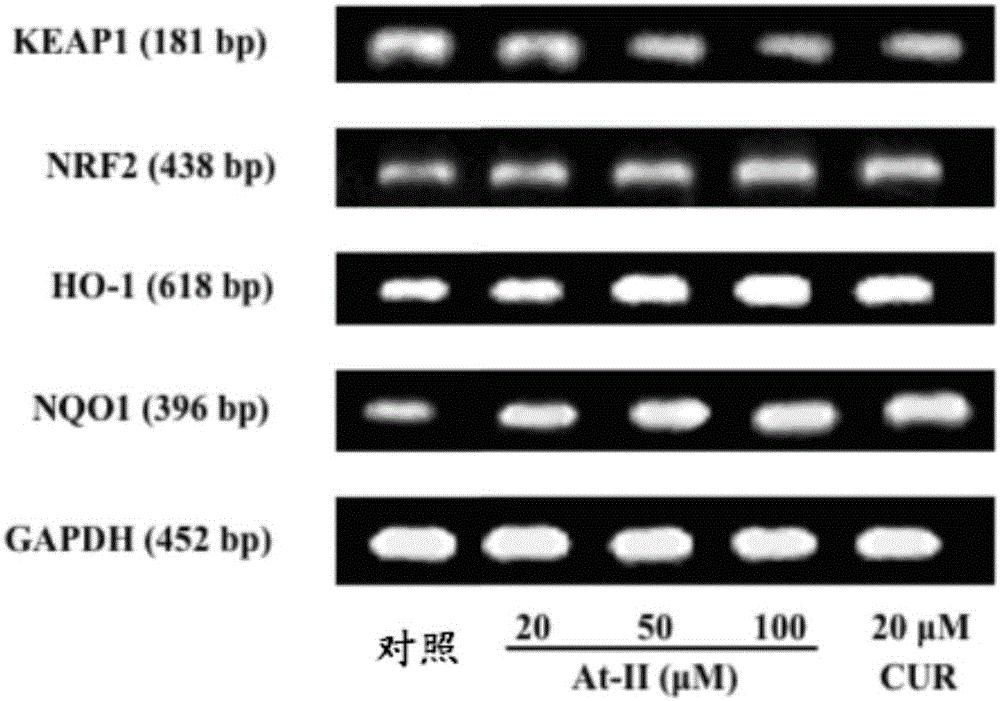
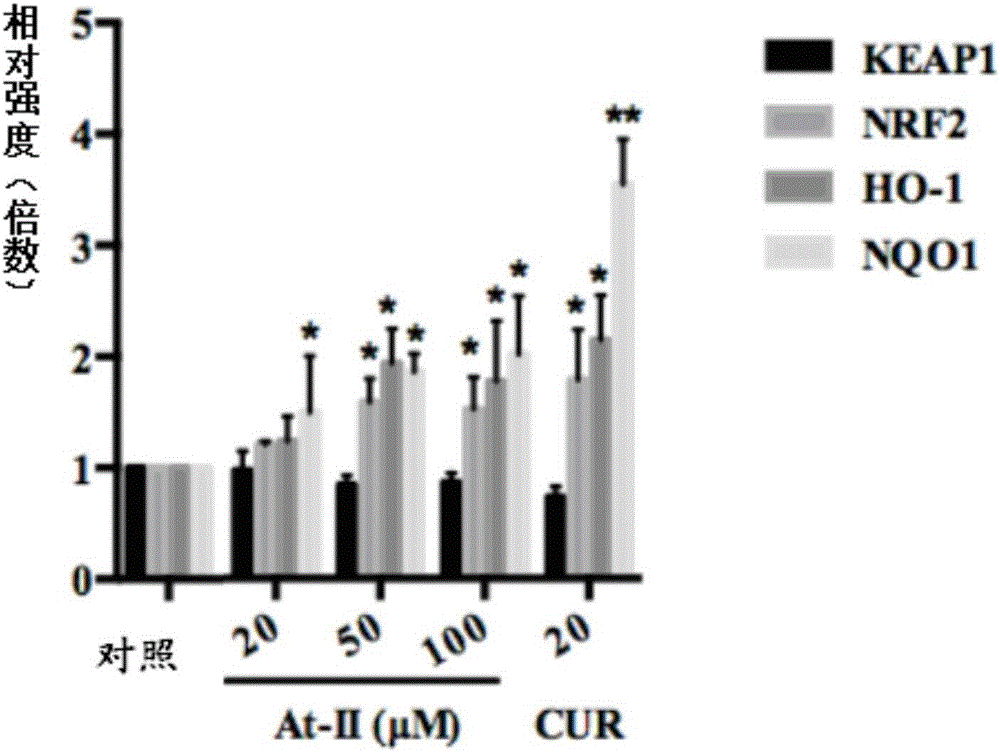
NRF2 activator compound, drugs and new application of atractylenolide II
InactiveCN106265633AReduce toxinsActive oxygen reductionOrganic active ingredientsNervous disorderDiseaseNrf2 activation
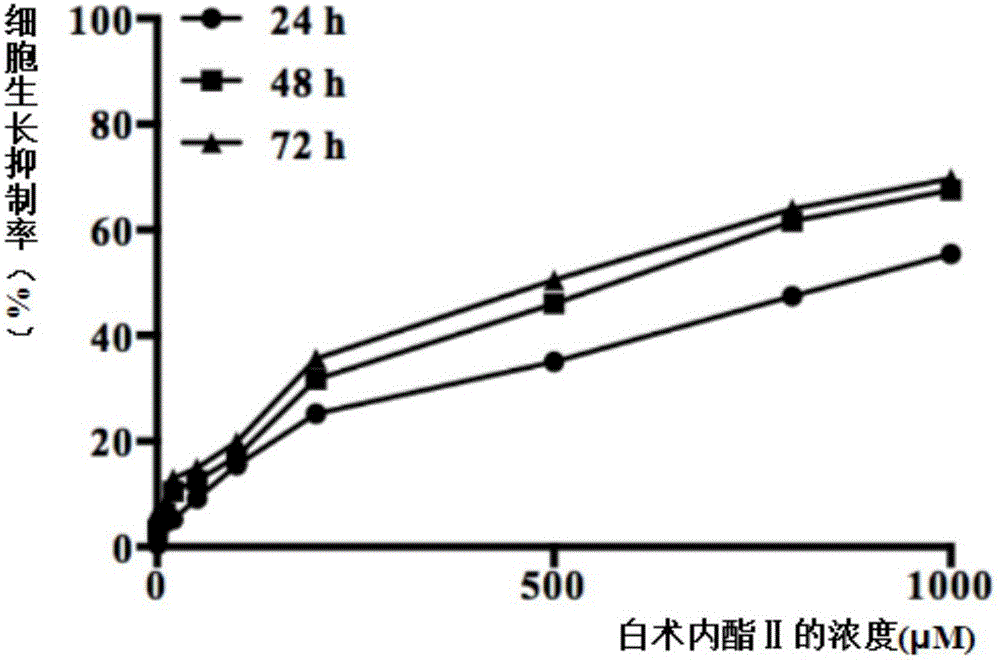
The invention discloses an NRF2 activator compound, drugs and new application of atractylenolide II, wherein the NRF2 activator compound is the atractylenolide II; the drugs are drugs for treating and preventing the diseases associated with NRF2 pathway regulation, and comprise the component atractylenolide II; the new application of the atractylenolide II is about the use of the atractylenolide II in preparing drugs or foods for treating or preventing the diseases associated with the NRF2 pathway regulation. Through a lot of exploration and research of the inventors, a plurality of compounds are tested and selected, and it is unexpectedly found that the atractylenolide II can be used as an effective NRF2 inducer. The experiments prove that the atractylenolide II can be used in the drugs for treating or preventing the diseases associated with the NRF2 pathway regulation. Particularly, the cell experiments and the animal experiments prove that the atractylenolide II has inhibitory effect on the growth of breast cancer cells.
Owner:SICHUAN UNIV
Characteristic spectrum construction method and quality detection method for bighead atractylodes rhizomes
InactiveCN109580857AHigh precisionOvercoming distractionsComponent separationAtractylenolide IIAdditive ingredient
The invention relates to the field of traditional Chinese medicine detection technology, in particular to a characteristic spectrum construction method and a quality detection method for bighead atractylodes rhizomes. According to the characteristic spectrum construction method for bighead atractylodes rhizomes, a characteristic spectrum of effective ingredients, namely atractylenolide I, atractylenolide II, atractylenolide III and atractylon of bighead atractylodes rhizome medicinal materials, bighead atractylodes rhizome herbal pieces and bighead atractylodes rhizome herbal pieces stir-bakedwith bran are analyzed through efficient liquid chromatographic analysis, chromatographic peaks of the characteristic spectrum are completely displayed, uniformity is good, the characteristic chromatographic peaks are completely separated, the quantity of impurities is small, a contrast characteristic spectrum is established according to the characteristic spectrum, and meanwhile the content of atractylenolide I and the content of atractylenolide III are determined. In combination with thin-layer chromatography identification and heavy metal and harmful element determination, the comprehensive quality detection method for bighead atractylodes rhizomes with effectiveness and safety is provided, so that quality detection on bighead atractylodes rhizomes, processed products of bighead atractylodes rhizomes and relevant products is realized. Moreover, the quality detection method has the advantages of being simple, fast, stable, reliable, high in precision, good in reproducibility, easy to grasp and the like.
Owner:BEIJING ZHONGYAN TONGRENTANG CHINESE MEDICINE R & D
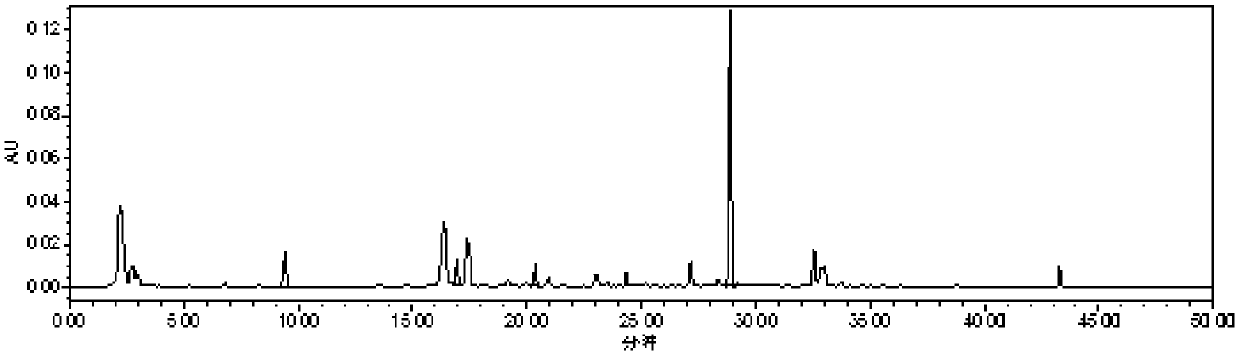
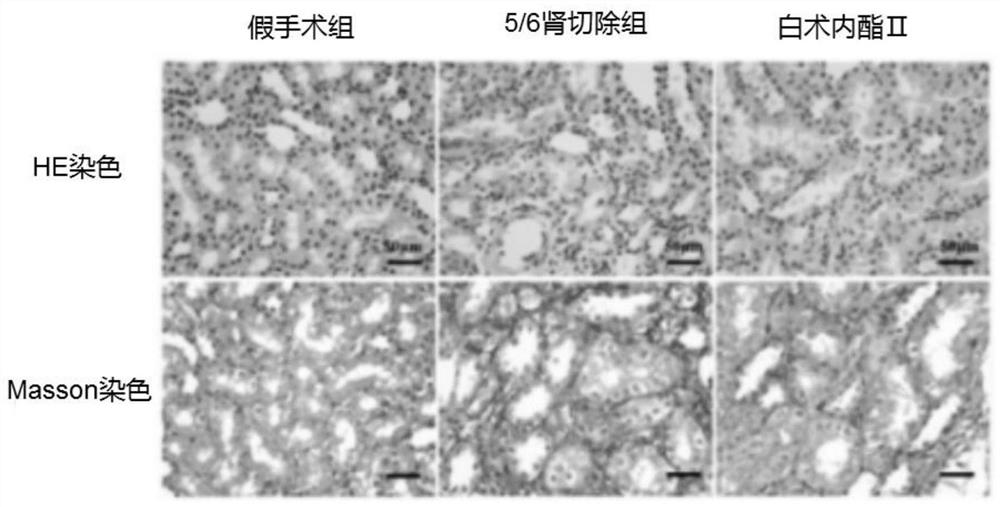
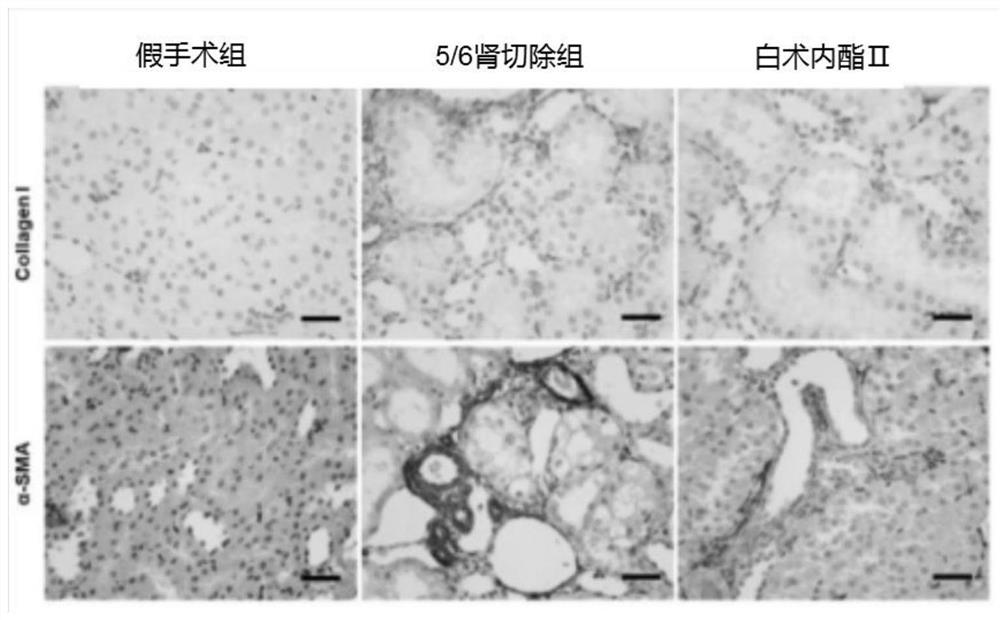
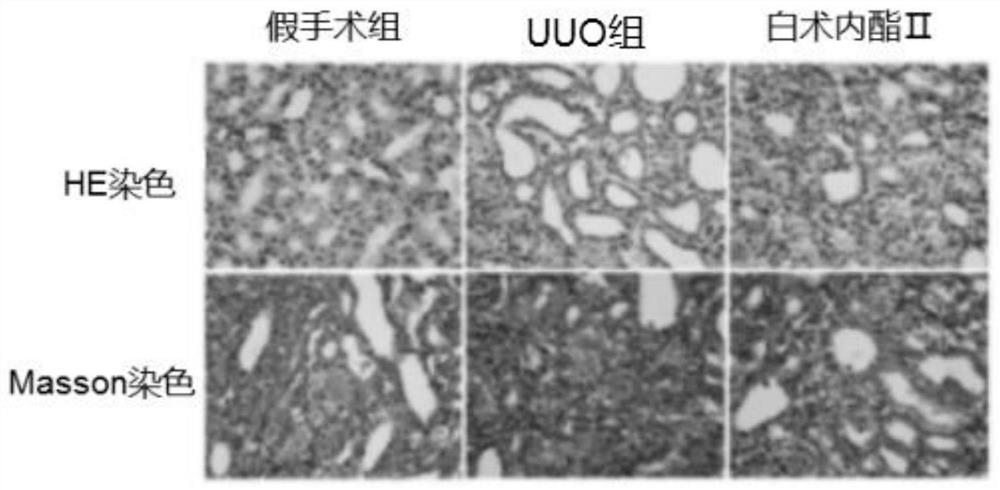
Application of atractylenolide II in preparation of anti-renal fibrosis drug and anti-renal fibrosis drug
ActiveCN111803488ASignificant anti-renal fibrosis effectNot easy to relapseOrganic active ingredientsUrinary disorderAtractylenolide IIAtractylenolide I
The invention relates to the field of medicines, and provides an application of atractylenolide II in preparation of an anti-renal fibrosis medicine, and an effective anti-renal fibrosis effect can beachieved by taking an atractylenolide II monomer as an effective component. The invention further provides an anti-renal fibrosis medicine which comprises the atractylenolide II and pharmaceutic adjuvants. Researches show that the atractylenolide II has a good effect of treating renal fibrosis.
Owner:ZHEJIANG CHINESE MEDICAL UNIVERSITY
Atractylenolide extract and application in CIK cell cryopreservation protection
InactiveCN107736338AEasy to prepareEasy to industrializeIon-exchange process apparatusOther chemical processesAtractylenolide IICryopreservation
The invention discloses atractylenolide extract and application in CIK cell cryopreservation protection. The atractylenolide extract is prepared by utilizing bighead atractylodes rhizome as a raw material and performing extracting and enriching, and the sum of contents of atractylenolide II and atractylenolide V in the extract is 90% or more, preferably 95% or more. The extracting and enriching step comprises S1 of acid addition for corrosion, S2 of ethanol extraction and S3 of macroporous resin enrichment. The atractylenolide extract disclosed by the invention can improve a CIK cell cryopreservation resuscitation rate, further avoids affecting killing activity of CIK cells to tumor cells, and can be prepared into a CIK cell cryopreservation solution for CIK cell cryopreservation, so as toguarantee that sufficient CIK cells can be fed back to patients after radiotherapy and chemotherapy.
Owner:南京盖斯夫医药科技有限公司
Method for detecting resistance marker in growing period of atractylodes macrocephala
The invention discloses a method for detecting a resistance marker in the growing period of atractylodes macrocephala. The method comprises: in the soil borne disease high-incidence season of atractylodes macrocephala, collecting infected plants and disease-resistant plants in natural state; also, employing a root cutting method to inoculate with sclerotium rolfsii mycelium; after the plants are infected and attacked, respectively collecting healthy plants and disease plants, and employing HPLC method to determine the level of main metabolites atractylenolides in the sample plants; comparing differences in groups, determining a significant-difference composition, and screening a metabolite marker correlated to atractylodes macrocephala resistance; and taking sclerotium rolfsii Sacc. as a test-receiving bacteria, and performing virulence tests on the composition, so as to prove the bacteriostatic efficacy of atractylenolide II and further to obtain the reisistance marker which is used for atractylodes macrocephala resistance evaluation, resistance variety breeding and other aspects.
Owner:ZHEJIANG FORESTRY UNIVERSITY
Quality detection method of Shouhui bowel-relaxing capsule
ActiveCN109507356AQuality assuranceComprehensive evaluationComponent separationAtractylenolide IIIAtractylenolide I
The invention discloses a quality detection method of a Shouhui bowel-relaxing capsule and belongs to the field of analysis of traditional Chinese medicinal preparations. The quality detection methodadopts a thin-layer chromatography to identify bighead atractylodes rhizome in the capsule and simultaneously adopts an HPLC to determine the contents of atractylenolide I, atractylenolide II and atractylenolide III in the capsule. The quality detection method disclosed by the invention is stable and reliable, strong in specificity, good in reproducibility and capable of fully and effectively controlling the quality of the euphorbia pulcherrima bowel-relaxing capsule, ensuring the safety and the effectiveness of clinical medication and better meeting the needs of patients and the market.
Owner:LUNAN PHARMA GROUP CORPORATION
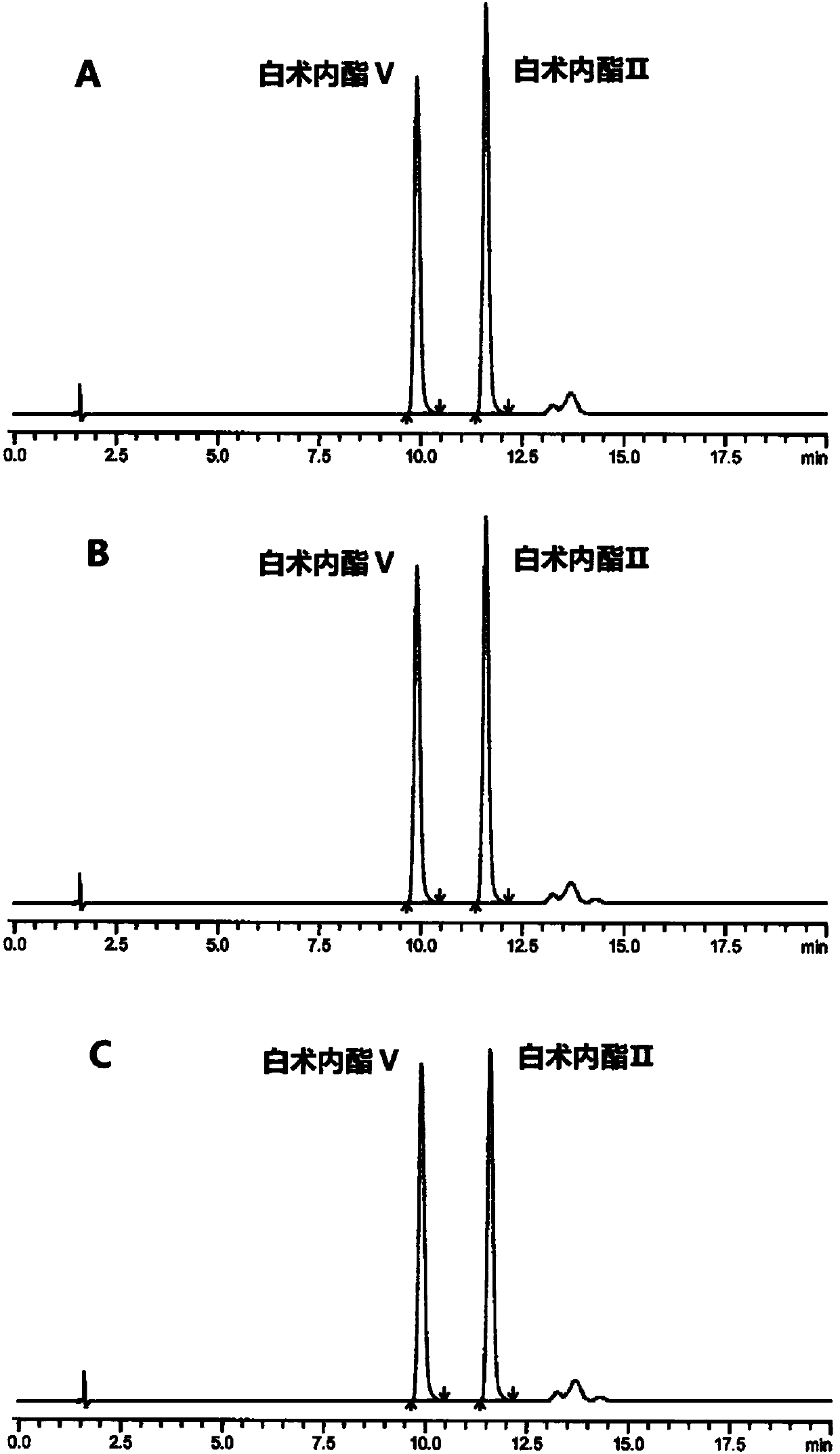
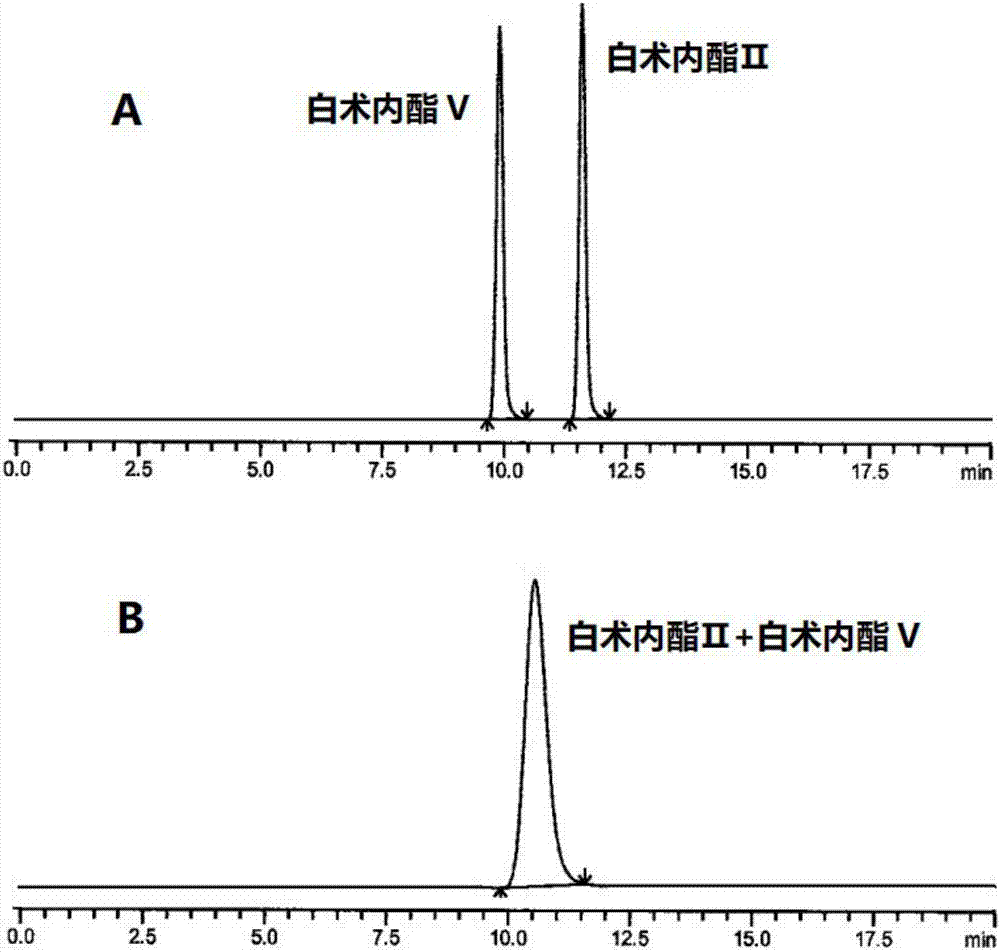
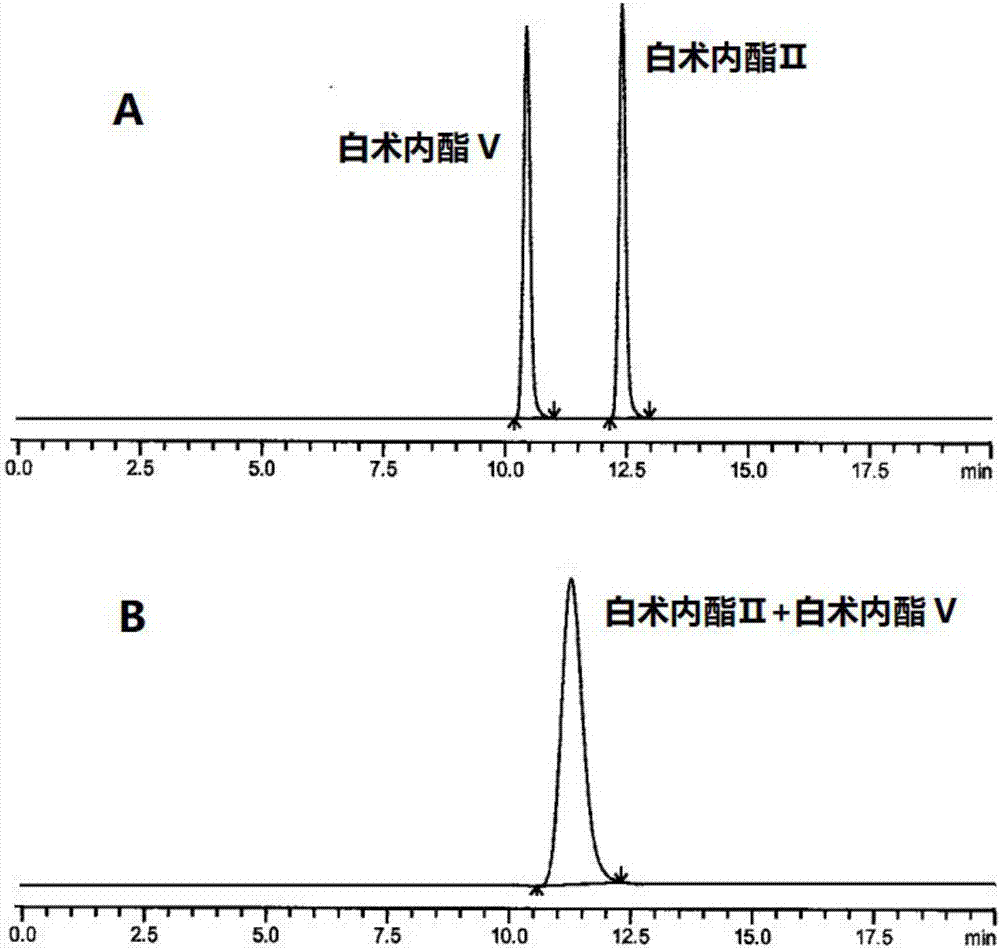
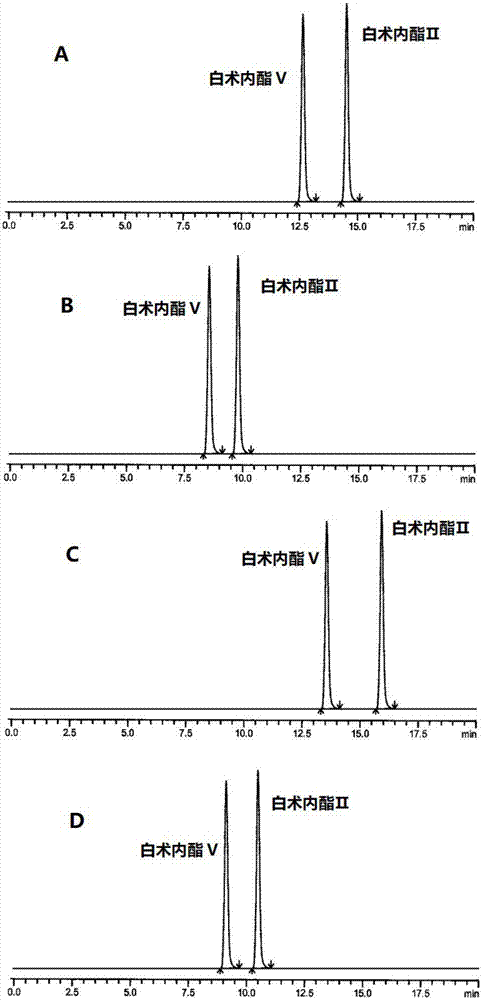
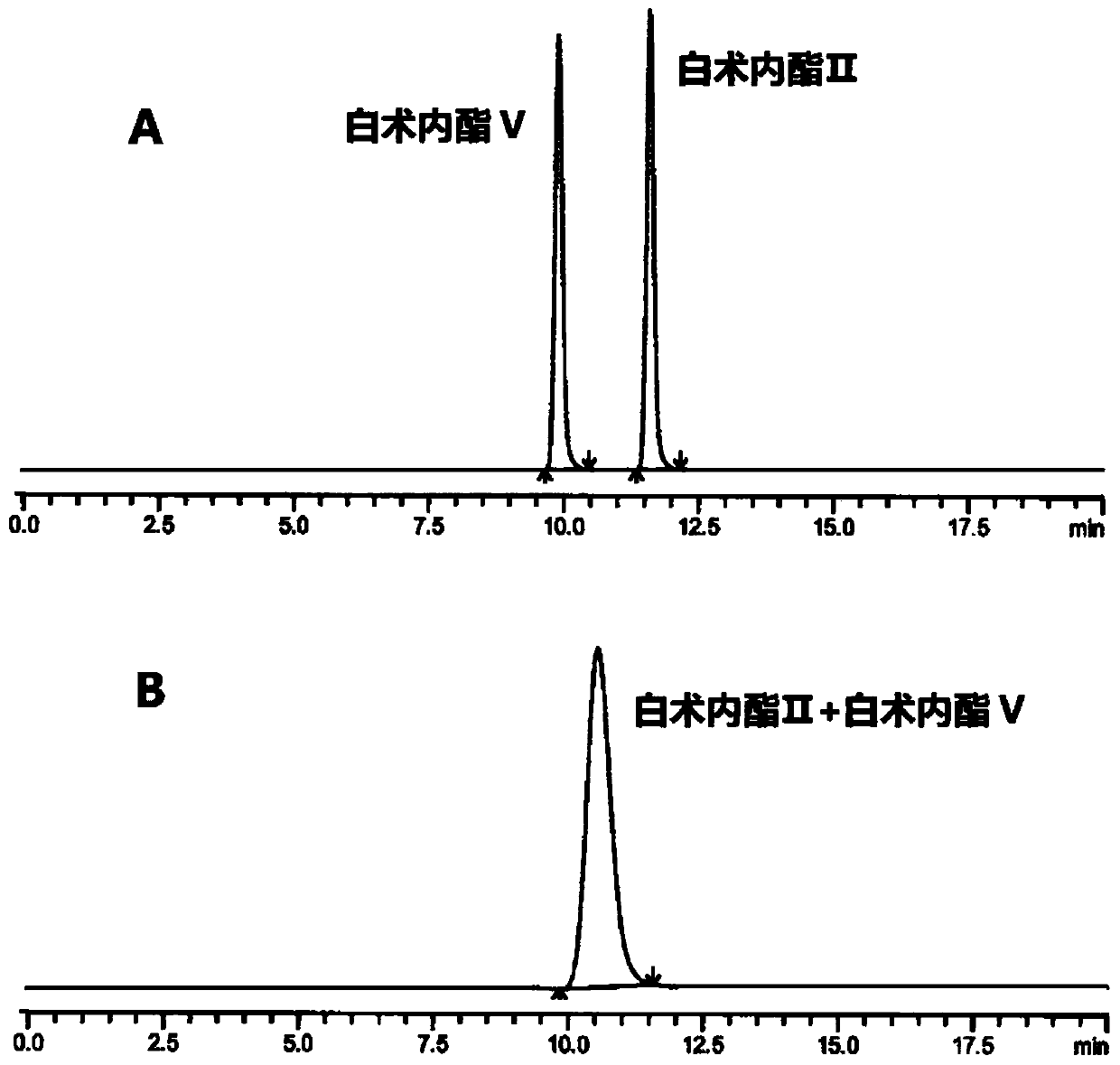
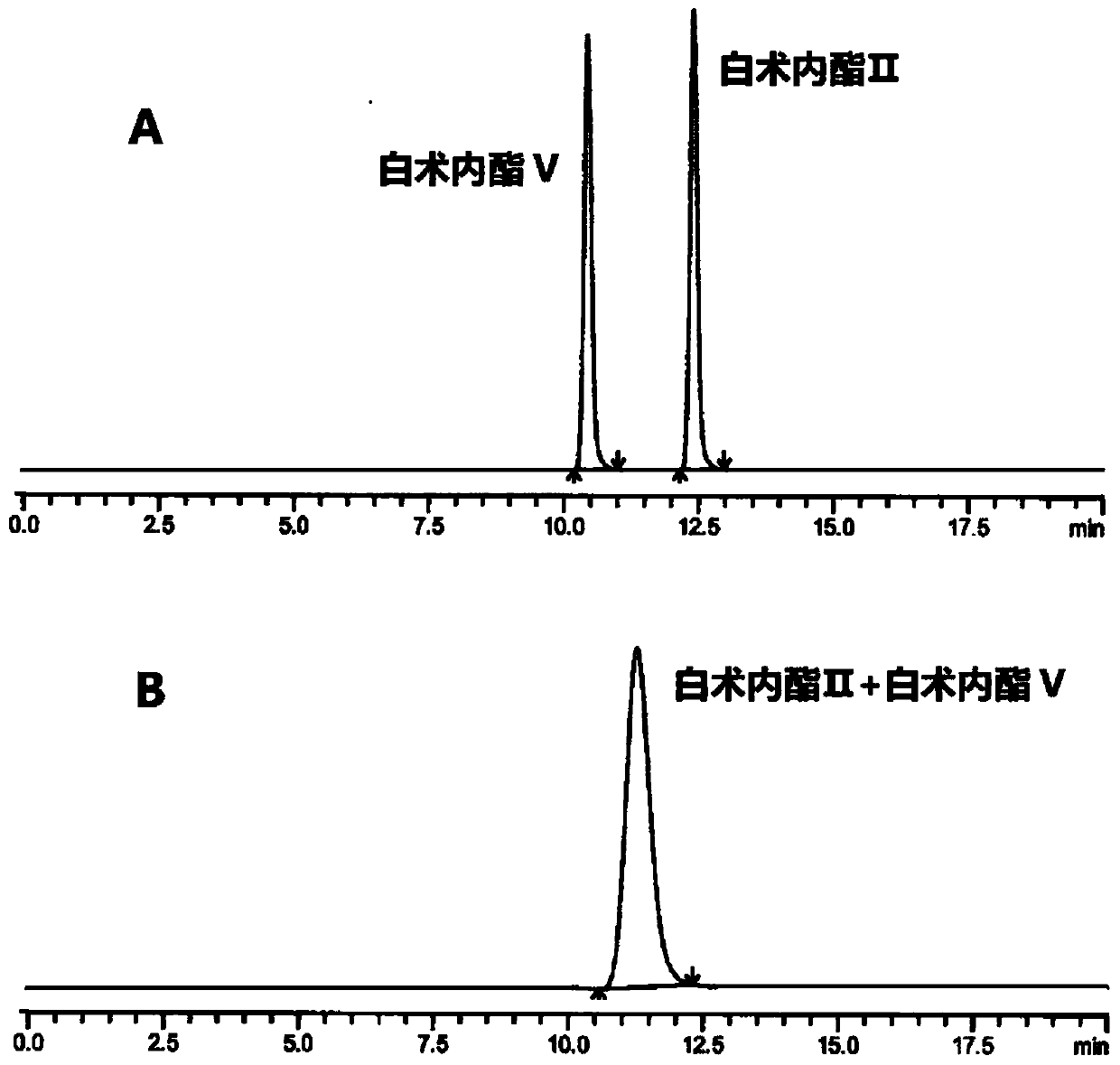
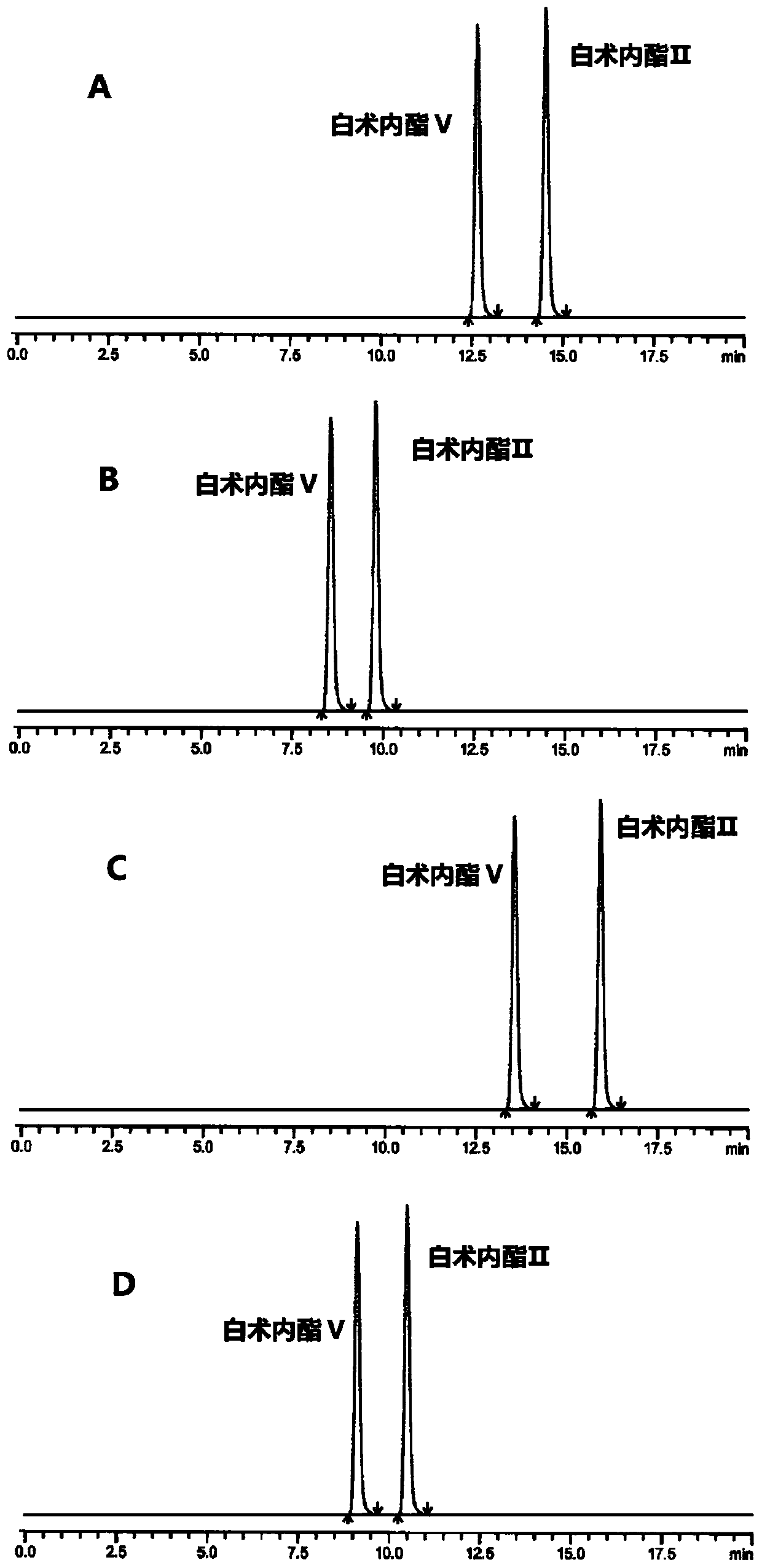
HPLC (High Performance Liquid Chromatography) method for separating and detecting atractylenolide II and atractylenolide V
ActiveCN107505416AEfficient separationGood repeatabilityComponent separationHplc methodAtractylenolide II
The invention discloses an HPLC (High Performance Liquid Chromatography) method for separating and detecting atractylenolide II and atractylenolide V. According to the method, taking octadecylsilane chemically bonded silica (Carbon 18) as a stationary phase, a trifluoroacetic acid-containing organic solvent aqueous solution is taken as a flowing liquid, specifically, the organic solvent aqueous solution is acetonitrile aqueous solution of which the acetonitrile volume percentage concentration is 34 to 38 percent and contains trifluoroacetic acid with the volume percentage concentration of 2.5 to 4.5 percent, or the organic solvent aqueous solution is methyl alcohol aqueous solution of which the methyl alcohol volume percentage concentration is 47 to 51 percent and contains the trifluoroacetic acid with the volume percentage concentration of 2 to 4 percent. According to the HPLC method provided by the invention, the atractylenolide II and the atractylenolide V can be separated effectively; the separation degree is greater than 1.5 and is accordance with regulations; the method only requires a common C18 chromatographic column and a common organic solvent; moreover, gradient elution is not required; the repeatability is high; the common C18 chromatographic column and the common organic solvent are easily transferred among different laboratories.
Owner:国科赛赋河北医药技术有限公司
Special fertilizer capable of improving quality of rhizoma atractylodis chinensis and fertilizing method
InactiveCN113321559AImprove qualityAlkali orthophosphate fertiliserAmmonium orthophosphate fertilisersAtractylenolide IIDiammonium phosphate
The invention relates to the technical field of agricultural planting, and in particular, relates to a special fertilizer capable of improving the quality of rhizoma atractylodis chinensis and a fertilizing method. The special fertilizer comprises a basic fertilizer, a topdressing fertilizer and a composite special fertilizer, wherein the basic fertilizer is formed by mixing urea, diammonium phosphate, ammonium sulfate and potassium sulfate, and the topdressing fertilizer is formed by mixing urea, ammonium sulfate and potassium sulfate; and the compound special fertilizer is prepared by processing potassium sulfate, monoammonium phosphate, ammonium bicarbonate, a root strengthening additive, clay, anti-caking agent paste and anti-caking agent powder. According to the method, the contents of atractydin, atractylone, beta-eudesmol and atractylenolide II can be remarkably increased, and the plant height, the leaf number, the stem and leaf fresh weight, the longest fibrous root length, the fibrous root fresh weight and the rhizome fresh weight of the rhizoma atractylodis chinensis are increased.
Owner:CHANGCHUN UNIV OF CHINESE MEDICINE
A method for detecting resistance markers in the growth stage of Atractylodes macrocephala
The invention discloses a method for detecting resistance markers of Atractylodes macrocephala during its growth period. The method is to collect susceptible plants and disease-resistant plants in the natural state during the high-incidence period of Atractylodes macrocephala soil-borne diseases; at the same time, adopt the method of root injury to inoculate the mycelia of Sphagnum albicans, and collect healthy and diseased plants respectively after the onset of infection, and use HPLC method The levels of major metabolites Atractylodes atractylodes lactones in the sample plants were measured, the differences between groups were compared, the components with significant differences were determined, and the marker metabolites related to the resistance of Atractylodes macrocephala were screened, and Sclerotium rolfsii Sacc.) is the test bacterium, and the toxicity test of this component is carried out to prove the antibacterial effect of Atractylodes lactone II, so as to obtain resistance markers, which are used for the evaluation of Atractylodes atractylodes resistance, the selection of resistant varieties, and the like.
Owner:ZHEJIANG FORESTRY UNIVERSITY
Quality evaluation method for Chinese herbal compound aconite lizhong decoction for treating gastric ulcer
ActiveCN109900847AQuick evaluationRapid determinationComponent separationAtractylenolide IIIChemical composition
The invention provides a quality evaluation method for Chinese herbal compound aconite lizhong decoction for treating gastric ulcer. The method defines content limits of 10 chemical components of benzoylaconitine, benzoylmesaconine, atractylenolide I, atractylenolide II, atractylenolide III, lobetyolin, liquiritin, liquiritigenin, 8-gingerol and 10-gingerol in the Chinese herbal compound aconite lizhong decoction; the content is determined through a liquid chromatography-mass spectrometry method; and meanwhile, the 10 chemical components are detected simultaneously. The method is used for evaluating the quality of the Chinese herbal compound aconite lizhong decoction for treating gastric ulcer. The content of the 10 chemical components in the Chinese herbal compound aconite lizhong decoction is detected through the liquid chromatography-mass spectrometry method, so that the 10 chemical components can be detected simultaneously and quantified through one injection; and the method is fast in determination, efficient, high in flexibility and simple and feasible in detection, and can quickly evaluate the quality of the Chinese herbal compound aconite lizhong decoction.
Owner:QIQIHAR UNIVERSITY
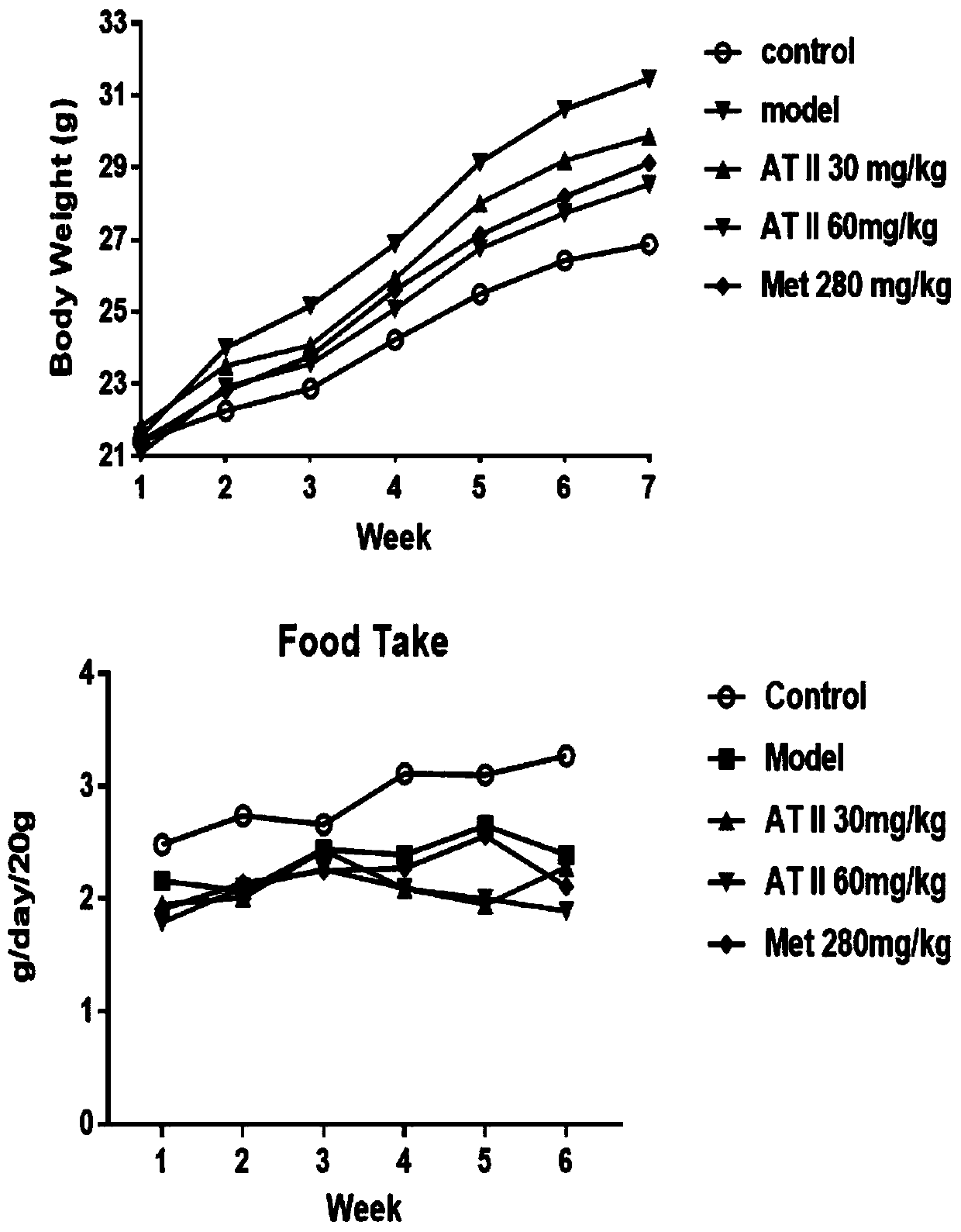
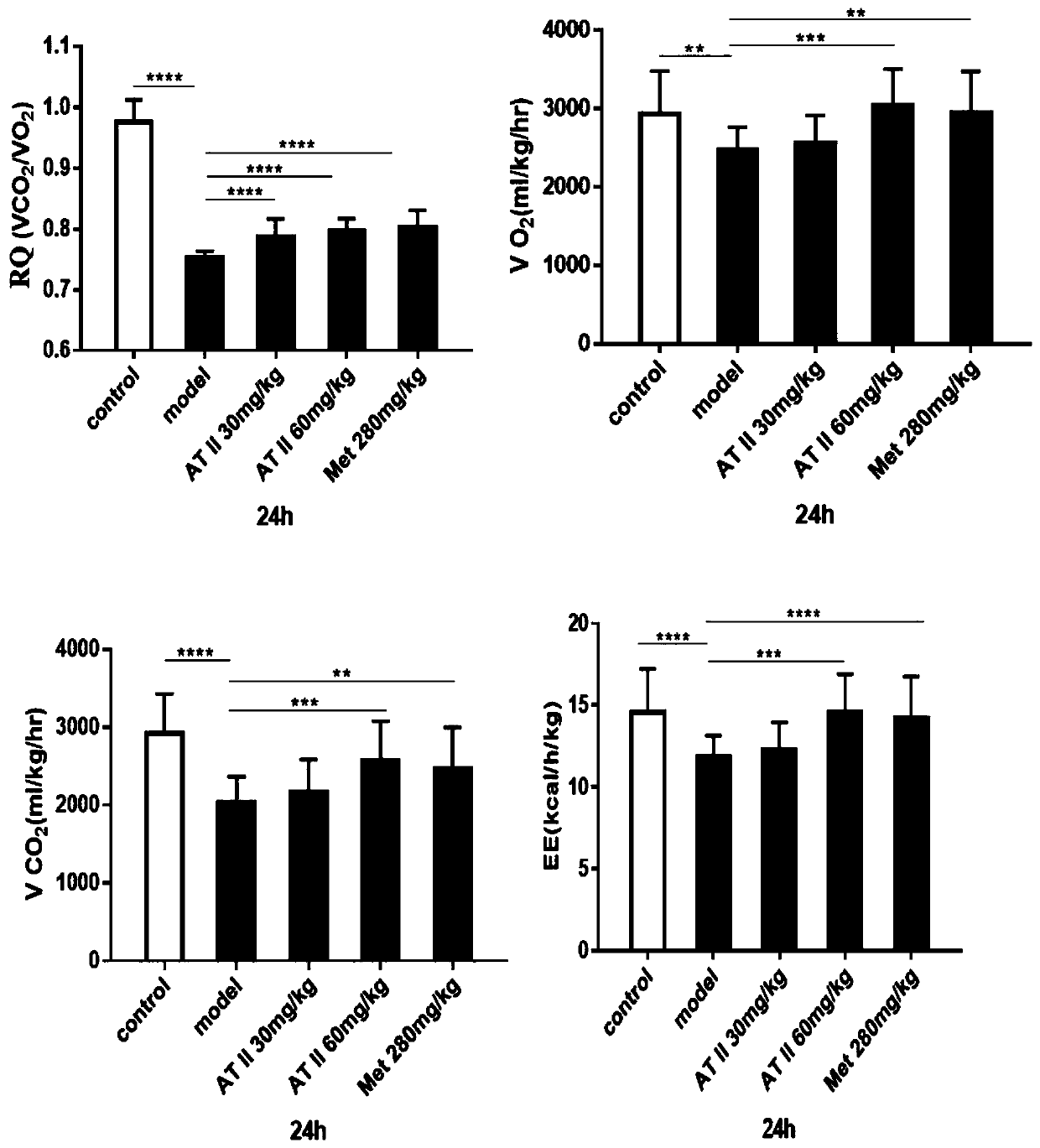
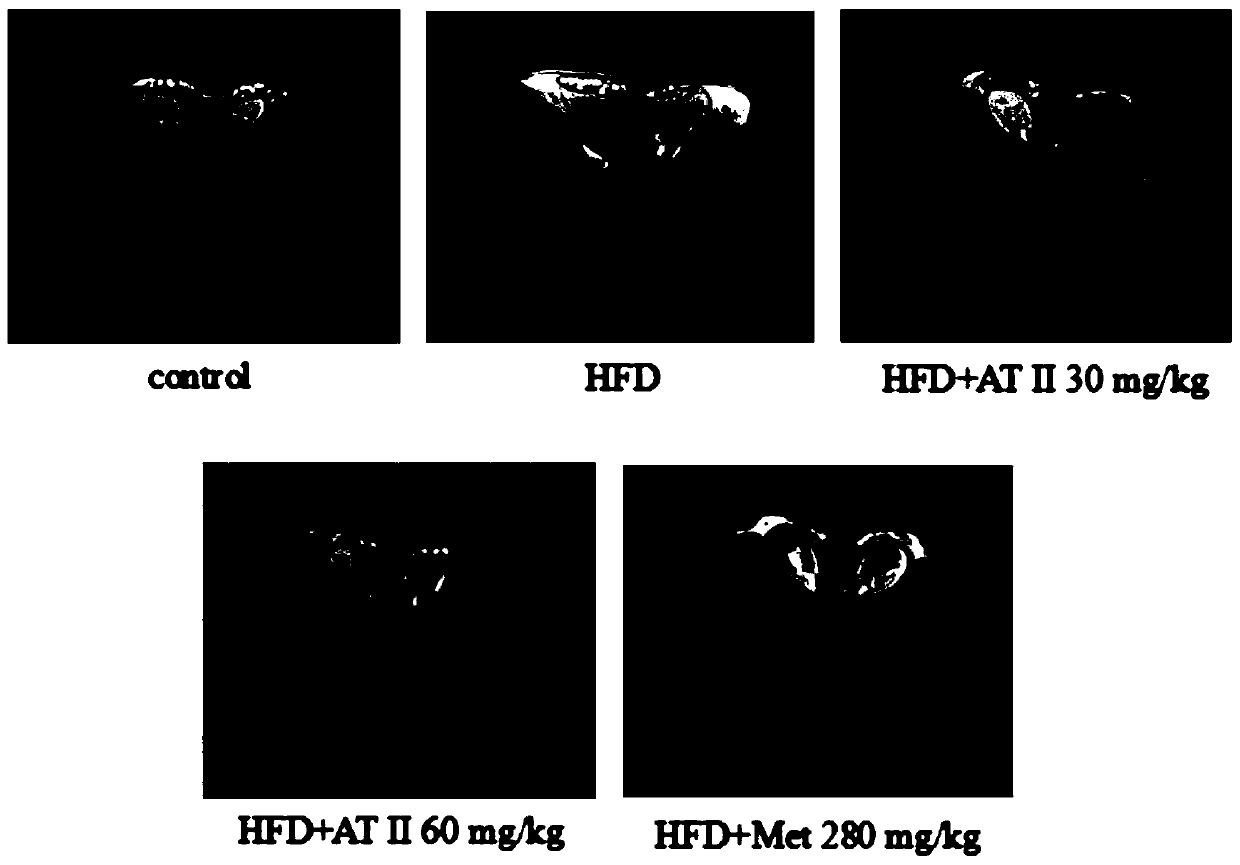
Application of atractylenolide II for preparing medicine capable of improving insulin resistance and glucose-lipid metabolism disorder caused by obesity
InactiveCN110548025AIncrease weightIncreased lipid dropletsOrganic active ingredientsMetabolism disorderDiseaseAtractylenolide II
The invention discloses application of atractylenolide II for preparing a medicine capable of improving insulin resistance and glucose-lipid metabolism disorder caused by obesity. After mice are fed in a high fat mode, the weights of the mice are increased, and respiratory quotient and energy consumption are obviously reduced, and thus, fat mice mainly take lipid metabolism as a main part insteadof glucose metabolism as the main part, fat tissues are pathologically changed and lipid droplets are increased, and meanwhile, some index detection of blood plasma and liver also proves diseases of liver damage and insulin resistance of the mice. Based on the application of the atractylenolide II disclosed by the invention, after the atractylenolide II is provided for treatment, the weights and the glucose-lipid metabolism of the fat mice are improved, the lipid droplets of the livers are reduced, and the insulin resistance is recovered. Therefore, because of the atractylenolide II, the insulin resistance and the glucose-lipid metabolism disorder caused by the obesity can be effectively improved.
Owner:CHINA PHARM UNIV
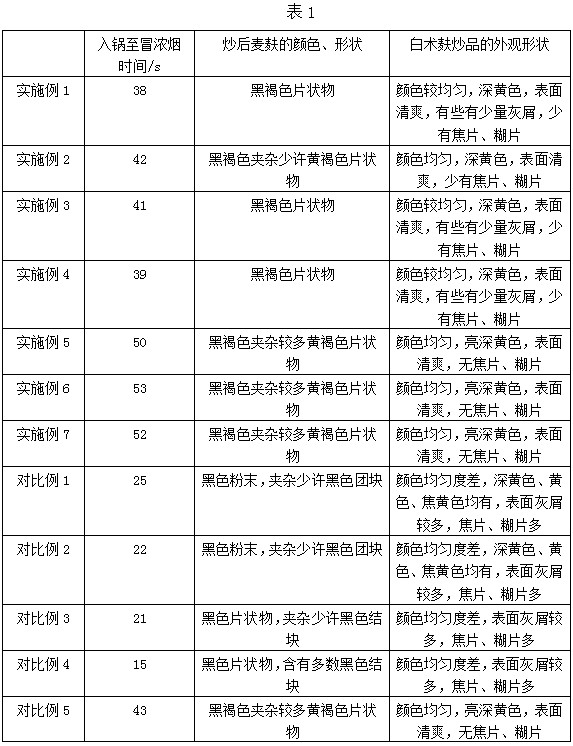
Processing technology of bran stir-fried rhizoma atractylodis macrocephalae decoction pieces
InactiveCN111700925AImprove appearance shapeUniform colorDigestive systemUnknown materialsAtractylenolide IIIAtractylenolide I
The invention discloses a processing technology of bran stir-fried rhizoma atractylodis macrocephalae decoction pieces and belongs to the technical field of preparation of traditional Chinese medicinedecoction pieces. The processing technology comprises the following steps: selection; cleaning, soaking and moistening: after cleaning mud on the surface of the rhizoma atractylodis macrocephalae, soaking the rhizoma atractylodis macrocephalae in drinking water at 30-50 DEG C, taking out and moistening the rhizoma atractylodis macrocephalae at 50-60 DEG C; cutting, drying; stir-frying: placing bran in a hot pan at 200-210 DEG C, after the bran smokes, adding the dried rhizoma atractylodis macrocephalae slices, stir-frying the rhizoma atractylodis macrocephalae slices for 25-3o min, and takingout of the rhizoma atractylodis macrocephalae slices until the surface of the rhizoma atractylodis macrocephalae slices turn yellowish brown and aroma overflows, wherein the particle size of the bran is larger than or equal to 40 meshes, the moisture conent is smaller than or equal to 10%, and the total ash of the bran is smaller than or equal to 6%; screening; and packaging. The prepared rhizoma atractylodis macrocephalae decoction pieces are uniform in color, have refreshing surfaces, have little ash and seldomly have burnt pieces and scorched pieces, have good appearance shapes and conform to the quality standard; and the content of atractylenolide I, atractylenolide II and atractylenolide III in rhizoma atractylodis macrocephalae decoction pieces is significantly increased, and the spleen and stomach strengthening functions of the rhizoma atractylodis macrocephalae decoction pieces are enhanced.
Owner:北京杏林药业有限责任公司
Preparation method of atractylenolide II
The invention discloses a preparation method of atractylenolide II. The preparation method comprises the following steps of S1, acid adding and corroding; S2, extracting by ethyl alcohol; S3, enriching by macroporous resin; S4, high-speed reverse chromatography purifying. The preparation method is characterized in that the acid adding and corroding are firstly performed under the acid condition, so that the content of atractylenolide II in the bighead atractylodes rhizome is obviously increased and is increased by 80 times or more; then, the atractylenolide II is selectively enriched by the macroporous adsorption resin, so as to obtain crude extract of the atractylenolide II; finally, the atractylenolide II with purity higher than 98% is obtained by the high-speed reverse chromatography purifying. The preparation method has the advantages that the utilization rate of the bighead atractylodes rhizome is improved, the enriching and purifying technology is simple, the repeated use of silicagel columns is not needed, and the preparation method is suitable for large-scale production.
Owner:广东正汇源实业有限公司
Mesenchymal stem cell proliferation and differentiation culture solution with atractylenolide II as nutrient factor
ActiveCN107574147APromote proliferationPromote osteogenic differentiationSkeletal/connective tissue cellsCulture fluidAtractylenolide II
The invention discloses a mesenchymal stem cell proliferation and differentiation culture solution with atractylenolide II as a nutrient factor. According to the culture solution, a DMEM / F12 (Dulbecco's Modified Eagle Medium) culture medium solution is adopted as a base culture solution, and the basic culture solution has atractylenolide II of an effective concentration; mesenchymal stem cells areadopted as mesenchymal stem cells; differentiation refers to osteogenesis differentiation; and the concentration of the atractylenolide II is 5-60mu m. Results show that the atractylenolide II is capable of promoting proliferation and osteogenesis differentiation of mesenchymal stem cells and can be adopted to prepare culture solutions for inducing osteogenesis differentiation of the mesenchymalstem cells, and thus the osteogenesis differentiation of the mesenchymal stem cells can be effectively increased.
Owner:HUNAN YUANPIN CELL TECH CO LTD
Method for determining multi-component content of Codonopsis pilosula based on HPLC multi-wavelength superposition
InactiveCN109839459AShorten the timeSave costsComponent separationAtractylenolide IAtractylenolide II
The invention discloses a method for determining multi-component content of Codonopsis pilosula based on HPLC multi-wavelength superposition in the technical field of traditional Chinese medicine detection. Lobetyolin, syringin, atractylenolide I, atractylenolide II and atractylodesin III are used as reference substances. Acetonitrile is used as mobile phase A, and water is used as mobile phase B.According to a gradient elution procedure, from 0 to 3min, the volume ratio of acetonitrile to water is 96.5:3.5; at 20min, the volume ratio of acetonitrile to water is 86:14; at 35min, the volume ratio of acetonitrile to water is 77:23; at 60min, the volume ratio of acetonitrile to water is 28:72; and at 76min, the volume ratio of acetonitrile to water is 5:95 by high performance liquid chromatography. The determining method provided by the invention can determine the content of various components at one time, which not only saves time and cost, but also makes a test result more stable and reliable under the same system condition.
Owner:ZUNYI MEDICAL UNIVERSITY
A kind of mesenchymal stem cell proliferation and differentiation culture medium
ActiveCN107574147BPromote proliferationPromote osteogenic differentiationSkeletal/connective tissue cellsAtractylenolide IIMesenchymal stem cell proliferation
The invention discloses a mesenchymal stem cell proliferation and differentiation culture solution with atractylenolide II as a nutrient factor. According to the culture solution, a DMEM / F12 (Dulbecco's Modified Eagle Medium) culture medium solution is adopted as a base culture solution, and the basic culture solution has atractylenolide II of an effective concentration; mesenchymal stem cells areadopted as mesenchymal stem cells; differentiation refers to osteogenesis differentiation; and the concentration of the atractylenolide II is 5-60mu m. Results show that the atractylenolide II is capable of promoting proliferation and osteogenesis differentiation of mesenchymal stem cells and can be adopted to prepare culture solutions for inducing osteogenesis differentiation of the mesenchymalstem cells, and thus the osteogenesis differentiation of the mesenchymal stem cells can be effectively increased.
Owner:HUNAN YUANPIN CELL TECH CO LTD
Determination method of various components in Yupingfeng preparation
ActiveCN105021751BQuality improvementImprove accuracyComponent separationAtractylenolide IIFiltration
The invention relates to a content determination method for multiple components in a Yupingfeng preparation. The method comprises the steps of: (1) taking the Yupingfeng preparation, crushing or not crushing it, then performing precise weighing, adding methanol to conduct reflux extraction for 1-3h, carrying out filtration and concentration, then adding methanol to a constant volume, thus obtaining a test solution; (2) precisely weighing the reference substance prim-o-glucosylcimifugin, calycosin-7-glucoside, cimifugin, 5-O-methylvisammioside, sec-o-glucosylhamaudol, calycosin, formononetin, atractylenolide III, atractylenolide I and atractylenolide II respectively, and adding methanol to perform dissolving to a constant volume, thus obtaining a mixed reference solution; (3) conducting determination: precisely sucking the test solution and the mixed reference solution respectively, injecting them into a high performance liquid chromatograph, carrying out gradient elution under certain mobile phase condition, and performing multi-wavelength simultaneous monitoring, thus obtaining the content. The method provided by the invention can accurately determine the content of 10 components in the Yupingeng preparation, and can objectively, comprehensively and sensitively reflect the quality condition of the Yupingfeng preparation.
Owner:GUANGZHOU XIANGXUE PHARMA CO LTD
A kind of preparation method of atractylodes lactone II
ActiveCN107556275BIncrease contentIncrease profitOrganic chemistryChromatographic separationAtractylenolide II
Owner:广东正汇源实业有限公司
Method for evaluating quality of atractylodes macrocephala koidz by quantitative analysis of multi-components by single marker
PendingCN113899844AImprove quality controlHigh detection sensitivityComponent separationAgainst vector-borne diseasesAtractylenolide IIIClinical efficacy
The invention provides a method for evaluating quality of atractylodes macrocephala koidz by a quantitative analysis of multi-components by single marker, wherein the method comprises the steps: performing quantitative analysis of multi-components by single marker by taking atractylenolide III which is low in cost and easy to obtain as an internal reference substance, and establishing relative retention time and relative correction factors between the atractylenolide III and atractylenolide II, atractylenolide I and atractylenone; and the contents of atractylenolide III, atractylenolide II, atractylenolide I and atractylenone in the atractylodes macrocephala koidz are calculated through correction factors, and the ultra-high performance liquid chromatography is adopted for determination. According to the method, the content of index components is calculated through relative correction factors and chromatographic peak positioning, the content of four components comprising atractylenolide III, atractylenolide II, atractylenolide I and atractylenone in atractylodes macrocephala koidz can be effectively detected at the same time, the cost can be saved, the operation is simplified, the efficiency is improved, the detection sensitivity is high, the stability is good, the determination result is accurate and reliable, and the method is of great significance to quality control of a large amount of atractylodes macrocephala koidz and guarantee of clinical effects of atractylodes macrocephala koidz.
Owner:ZHEJIANG SHOUXIANGU BOTANICAL DRUG INST CO LTD +2
Rapid thin-layer identification method for lyophilized powder of Linggui Zhugan decoction
PendingCN114689783ARealize authenticationLow costComponent separationMedicinal herbsAtractylenolide II
The invention discloses a rapid thin-layer identification method for lyophilized powder of Linggui curcuma zedogan decoction, which comprises the following steps: taking lyophilized powder of Linggui curcuma zedogan decoction, adding a trichloromethane-methanol mixed solution, carrying out ultrasonic treatment, filtering, drying by distillation, and adding methanol into residues to obtain a test solution; preparing a reference medicinal material solution from a poria cocos reference medicinal material by the same method; taking cinnamic acid, atractylenolide II and liquiritin reference substances, and adding methanol to prepare a reference substance solution; the reference medicinal material solution, the cinnamic acid reference substance solution and the atractylenolide II reference substance solution are sucked, and spots with the same color are shown at the positions corresponding to the cinnamic acid reference substance chromatogram and the poria cocos reference medicinal material in the test substance chromatogram; in the chromatogram of the sample to be tested, fluorescent spots with the same color are displayed under an ultraviolet lamp at the position corresponding to the chromatogram of the atractylenolide II reference substance; the method comprises the following steps: sucking a liquiritin reference substance solution and a test substance solution, and in a test substance chromatogram, displaying fluorescent spots with the same color at a position corresponding to a liquiritin reference substance chromatogram under an ultraviolet lamp.
Owner:SICHUAN NEO GREEN PHARMA TECH DEV
Method for determining contents of atractylenolide II and atractylenolide III in atractylodes macrocephala medicinal material
PendingCN112964805AGood peak shapeGood reproducibilityComponent separationMedicinal herbsO-Phosphoric Acid
The invention relates to a method for determining the contents of atractylenolide II and atractylenolide III in an atractylodes macrocephala medicinal material, which comprises the following steps of: taking the atractylodes macrocephala medicinal material, extracting by using 70% methanol, carrying out gradient elution by using an acetonitrile-0.1% phosphoric acid water system, and determining the contents of atractylenolide II and atractylenolide III in the atractylodes macrocephala medicinal material. According to the method, on the basis of a rhizoma alismatis decoction detection method, through optimization of a series of research on mobile phase proportion gradient, detection wavelength, an extraction solvent, an extraction mode, extraction time and a chromatographic column, the result shows that the peak time is short, the peak shape is good, the separation degree and the peak purity are qualified, the accuracy is high and the stability is good, the operation is simple, convenient and rapid, the quality detection method of the atractylodes macrocephala medicinal material is perfected, so that the quality is better controlled.
Owner:青海普兰特药业有限公司
A process for separating and purifying atractylolide i and atractylolide ii in Codonopsis pilosula with macroporous adsorption resin
ActiveCN106699711BLarge adsorption capacityImprove adsorption capacityOrganic chemistryAlcoholCodonopsis
The invention relates to a technology for separating and purifying atractylenolide I and atractylenolide II in radix codonopsis by using macroporous adsorption resin, belonging to the technical field of natural pharmaceutical chemistry. The technology comprises the following steps: 1, extracting the radix codonopsis by using an alcohol solution to obtain alcohol extract; carrying out vacuum concentration on the alcohol extract to dry, wherein the obtained solid is an extract of the radix codonopsis, and adding water into the solid for dissolving so as to obtain an extract solution; 2, absorbing the extract solution by using the macroporous adsorption resin DM130, and then desorbing by using the alcohol solution to obtain a desorbed solution; 3, carrying out vacuum concentration on the desorbed solution to dry so as to obtain an extract containing the atractylenolide I and the atractylenolide II. By utilizing the macroporous adsorption resin DM130 to separate and purify the atractylenolide I and the atractylenolide II in the radix codonopsis, the technology has the characteristics of being rapid in absorption speed, high in desorption rate, large in absorption capacity, high in elution rate, and the like, and the product is stable in desorption rate, so that the technology provided by the invention is an effective technology for deep utilization of the radix codonopsis.
Owner:GANSU UNIV OF CHINESE MEDICINE
Method for inducing ipsc to be differentiated into myocardial cells
PendingCN114525242AShort differentiation timeHigh differentiation efficiencyCulture processSkeletal/connective tissue cellsAtractylenolide IIAtractylenolide I
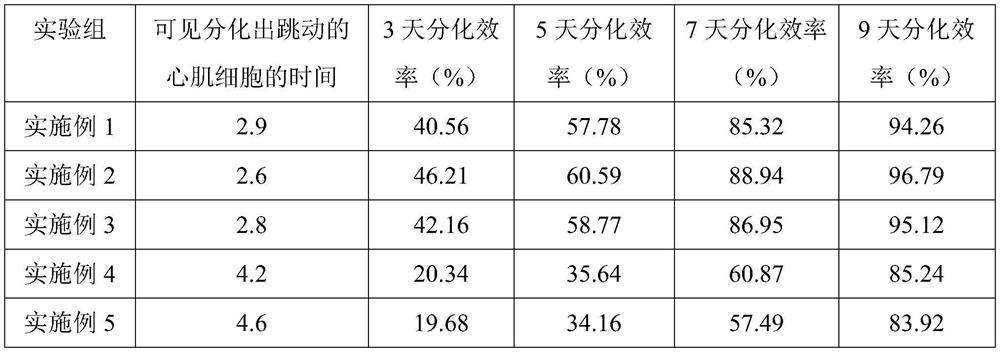
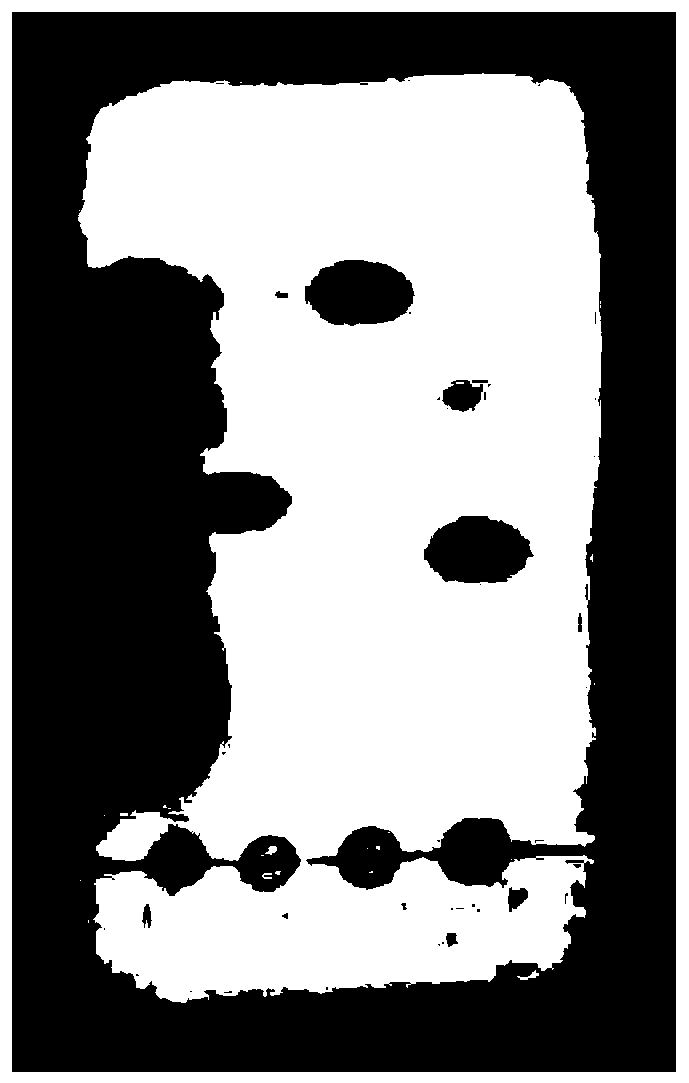
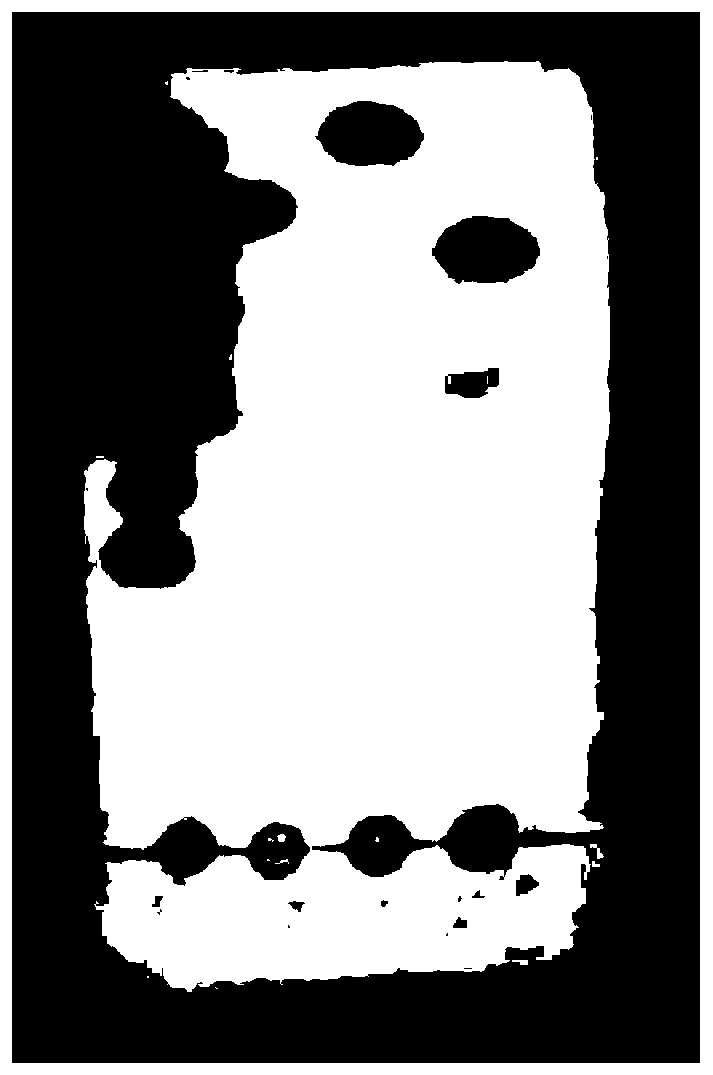
The invention discloses a method for inducing ipsc to be differentiated into myocardial cells, and relates to the technical field of biology. The method comprises the following steps: S1, carrying out ipsc cell culture; and S2, inducing the ipsc to be differentiated into myocardial cells. The method comprises the following steps: culturing ipsc by using a stem cell culture medium until the ipsc grows to 80%, culturing by using a mixed culture medium, inducing the ipsc to differentiate into mesoderm precursor cells, culturing by using an inhibitor culture medium, differentiating the cells into myocardial-like precursor cells, and culturing by using a maintenance culture medium to obtain myocardial cells. The mixed culture medium is a basic culture medium containing Chir99021 and atractylenolide II, and the basic culture medium is an RPMI 1640 culture medium containing a B27 additive and LAA2P; the inhibitor culture medium is a basic culture medium containing Wnt-C59; the maintenance culture medium is a basic culture medium. The method for inducing the ipsc to be differentiated into the myocardial cells has the advantages that the differentiation time is short (jumping myocardial cells can be differentiated in 3 days), and the differentiation efficiency is high (the differentiation efficiency reaches up to 85% in 7 days).
Owner:武汉百翼生物科技有限公司
Quality testing method of Shouhui Tongbian Capsules
ActiveCN109507356BQuality assuranceComprehensive evaluationComponent separationAtractylenolide IIIAtractylenolide II
The invention discloses a quality detection method of a Shouhui bowel-relaxing capsule and belongs to the field of analysis of traditional Chinese medicinal preparations. The quality detection methodadopts a thin-layer chromatography to identify bighead atractylodes rhizome in the capsule and simultaneously adopts an HPLC to determine the contents of atractylenolide I, atractylenolide II and atractylenolide III in the capsule. The quality detection method disclosed by the invention is stable and reliable, strong in specificity, good in reproducibility and capable of fully and effectively controlling the quality of the euphorbia pulcherrima bowel-relaxing capsule, ensuring the safety and the effectiveness of clinical medication and better meeting the needs of patients and the market.
Owner:LUNAN PHARMA GROUP CORPORATION
A hplc method for separating and detecting atractyloid ii and atractyloid v
ActiveCN107505416BEfficient separationGood repeatabilityComponent separationHplc methodAtractylenolide II
The invention discloses an HPLC (High Performance Liquid Chromatography) method for separating and detecting atractylenolide II and atractylenolide V. According to the method, taking octadecylsilane chemically bonded silica (Carbon 18) as a stationary phase, a trifluoroacetic acid-containing organic solvent aqueous solution is taken as a flowing liquid, specifically, the organic solvent aqueous solution is acetonitrile aqueous solution of which the acetonitrile volume percentage concentration is 34 to 38 percent and contains trifluoroacetic acid with the volume percentage concentration of 2.5 to 4.5 percent, or the organic solvent aqueous solution is methyl alcohol aqueous solution of which the methyl alcohol volume percentage concentration is 47 to 51 percent and contains the trifluoroacetic acid with the volume percentage concentration of 2 to 4 percent. According to the HPLC method provided by the invention, the atractylenolide II and the atractylenolide V can be separated effectively; the separation degree is greater than 1.5 and is accordance with regulations; the method only requires a common C18 chromatographic column and a common organic solvent; moreover, gradient elution is not required; the repeatability is high; the common C18 chromatographic column and the common organic solvent are easily transferred among different laboratories.
Owner:国科赛赋河北医药技术有限公司
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com