Method for inducing ipsc to be differentiated into myocardial cells
A cardiomyocyte and cell technology, applied in the biological field, can solve the problems of long differentiation time and low differentiation efficiency, and achieve the effect of short differentiation time and high differentiation efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
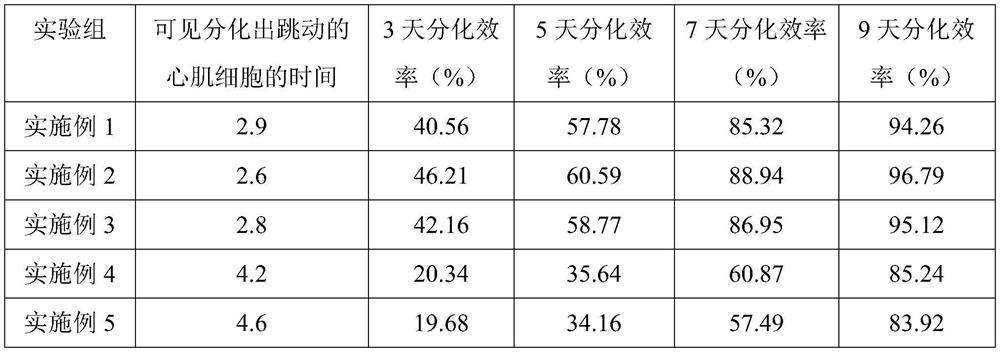
Embodiment 1
[0023] A method for inducing the differentiation of iPSCs into cardiomyocytes
[0024] S1 ipsc cell culture: Use matrigel working solution diluted 1:150 to completely cover the petri dish, and let stand at 37°C overnight. The iPSC clones are cultured in the Matrigel-coated petri dish until the degree of incubation reaches 70% to 80%. When culturing, remove the stem cell culture medium, then wash 1-3 times with PBS solution without calcium and magnesium, subculture with 0.5mmol / L EDTA, shake the culture flask gently during culture, so that EDTA covers all the cell surface, wait for the cell layer Slightly loose, when the "film" phenomenon can be observed with the naked eye, pour out the EDTA, continue to act for 2-3 minutes, shake gently, the cell layer can fall off from the bottle wall in flakes with the remaining EDTA, when it is passed through a microscope When it is found that the cells shrink and become round, and the intercellular space increases, the digestion should be ...
Embodiment 2
[0031] A method for inducing the differentiation of iPSCs into cardiomyocytes
[0032] S1 ipsc cell culture: use 1:200 diluted matrigel working solution to completely cover the petri dish, and let stand at 37°C overnight. The iPSC clones are cultured in the Matrigel-coated petri dish until the degree of rotation reaches 70% to 80%. During the culture, the stem cell culture medium was aspirated, then washed 1-3 times with PBS solution without calcium and magnesium, and subcultured with 0.5mmol / L EDTA. Gently shake the culture flask during culture, so that EDTA covers all the cell surface, wait for the cell layer Slightly loose, when the "film" phenomenon can be observed with the naked eye, pour out the EDTA, continue to act for 2-3 minutes, shake gently, the cell layer can fall off from the bottle wall in flakes with the remaining EDTA, when it is passed through a microscope When it is found that the cells shrink and become round, and the intercellular space increases, the dige...
Embodiment 3
[0039] A method for inducing the differentiation of iPSCs into cardiomyocytes
[0040] S1 ipsc cell culture: Use matrigel working solution diluted 1:250 to completely cover the petri dish, and let stand at 37°C overnight. The iPSC clones are cultured in the Matrigel-coated petri dish until the degree of rotation reaches 70% to 80%. When cultured, the stem cell culture medium was aspirated, then washed 1-3 times with calcium and magnesium-free PBS solution, and subcultured with 0.5 mmol / L EDTA. Gently shake the culture flask during culture to make EDTA cover all cell surfaces, and wait for the cell layer Slightly loose, when the "film" phenomenon can be observed with the naked eye, pour out the EDTA, continue to act for 2-3 minutes, shake gently, the cell layer can fall off from the bottle wall in flakes with the remaining EDTA, when it is passed through a microscope When it is found that the cells shrink and become round, and the intercellular space increases, the digestion sh...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com