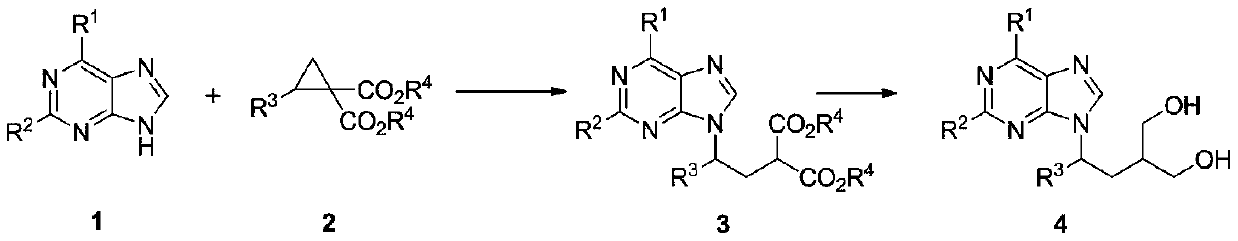
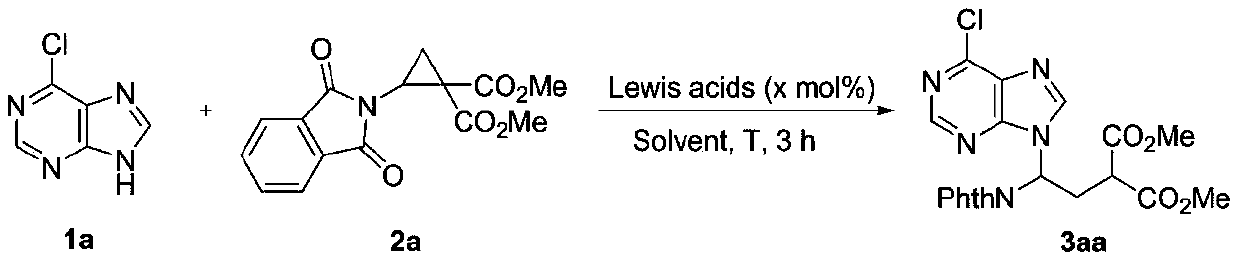Method for synthesizing penciclovir analogue
A technology for penciclovir and analogs, applied in the field of pharmaceutical intermediate synthesis, can solve problems such as insufficient research, and achieve the effects of high selectivity and excellent yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022]
[0023]
[0024] [a] Unless otherwise noted, the reaction conditions were as follows: 1a0.1mmol), catalyst (x mol%), MS(30mg),solvent(1mL)for 3h.[b]Isolated yield.[c]Reaction time:0.5h.
[0025] In the screening process of reaction conditions, the influence of different Lewis acid catalysts on the reaction was first investigated (entries1-5), and finally the Sc(OTf) 3 as the best catalyst. At the same time, considering the influence of solvent, temperature, the ratio of reactants and the amount of catalyst on the reaction, DCE was finally selected as the solvent, the reaction temperature was 70°C, the ratio of reactants was 1a:2a=1:2, and the amount of catalyst was 20mol%.
[0026] Investigation of reaction conditions (taking entry 13 as an example):
[0027] In the reaction tube, 6-chloropurine 1a (0.1mmol, 15.4mg), amino cyclopropane methyl ester 2a (0.2mmol, 60.6mg), Sc(OTf) 3 (20mol%, 9.8mg) and MS (30 mg) was added to the reaction tube, and 1 mL of 1,2...
Embodiment 2
[0034] In a reaction tube, 2-fluoro6-chloropurine 1c (0.1mmol, 17.1mg), amino cyclopropane methyl ester 2a (0.2mmol, 60.6mg), Sc(OTf) 3 (20mol%, 9.8mg) and MS (30 mg) was added to the reaction tube, and 1 mL of 1,2-dichloroethane was added to the reaction system, and the reaction tube was placed in an oil bath at 70° C. for 0.5 h. After the reaction was terminated by TLC detection, the target compound 3ca was obtained by concentration and column chromatography with a yield of 90%.
[0035] 3ca Colorless oil, 42.7mg, 90% yield. 1 H NMR (600MHz, CDCl 3 )δ8.71(s,1H),7.91-7.88(m,2H),7.81-7.78(m,2H),6.95-6.92(m,1H),3.73(s,3H),3.68(s,3H) ,3.40-3.35(m,2H),3.27-3.22(m,1H). 13 C NMR (150MHz, CDCl 3 )δ168.0, 167.9, 166.7, 158.4, 156.9, 153.33, 153.26, 153.21, 153.15, 145.11, 145.09, 135.3, 131.0, 129.64, 129.60, 124.4, 57.0, 53.43, 53.35, 48. 20 h 15 o 6 N 5 ClFNa[M+Na] + 498.0587,found 498.0592.
Embodiment 3
[0037] In the reaction tube, 6-methoxypurine 1e (0.1mmol, 15.0mg), amino cyclopropane methyl ester 2a (0.2mmol, 60.6mg), Sc (OTf) 3 (20mol%, 9.8mg) and MS (30 mg) was added to the reaction tube, and 1 mL of 1,2-dichloroethane was added to the reaction system, and the reaction tube was placed in an oil bath at 70° C. for 0.5 h. After the reaction was terminated by TLC detection, the target compound 3ea was obtained by concentration and column chromatography with a yield of 67%.
[0038] 3ea Colorless oil, 30.6mg, 67% yield. 1 H NMR (400MHz, CDCl 3)δ8.58(s,1H),8.53(s,1H),7.90-7.85(m,2H),7.78-7.73(m,2H),7.08-7.03(m,1H),4.16(s,3H) ,3.72(s,3H),3.67(s,3H),3.40-3.24(m,3H). 13 C NMR (100MHz, CDCl 3 )δ168.2, 168.1, 166.9, 161.3, 153.0, 151.7, 141.4, 135.0, 131.2, 124.2, 120.7, 56.5, 54.4, 53.3, 53.2, 48.5, 31.0.HRMScalcd for C 21 h 20 o 7 N 5 [M+H] + 454.1357, found 454.1357.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com