External electron donor for olefin polymerization catalyst, catalyst system and preparation method of polyolefin
An external electron donor and olefin polymerization technology, which is applied in the field of olefin polymerization catalyst system to prepare polyolefin, can solve the problems of high branching efficiency, large amount of monomer, low long-chain branching efficiency, etc., and achieves increased melt elasticity, The effect of improving the efficiency of long chain branching
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0040] The third aspect of the present invention provides a method for preparing polyolefin, wherein the method comprises: performing bulk polymerization, gas phase polymerization or slurry polymerization of olefin monomers in the presence of the above catalyst system.
[0041] In the present invention, the olefin may be, but not limited to: ethylene or propylene.
[0042] In the present invention, bulk polymerization refers to liquid phase bulk polymerization, for example, liquid propylene is subjected to bulk polymerization in the presence of the above-mentioned catalyst system.
[0043] In the present invention, gas-phase polymerization refers to the polymerization of olefin monomers in a gasified state, for example, the polymerization of gaseous propylene is catalyzed in the presence of the above-mentioned catalyst system.
[0044] In the present invention, slurry polymerization refers to the polymerization of olefin monomers catalyzed by the above-mentioned catalyst syste...
preparation example 1
[0055] (1) Preparation of Dihexenyldiethoxysilane
[0056] The steps are: under the protection of nitrogen, the divinyl silane (Hexe 2 SiH 2 , 3.92g, 20mmol), absolute ethanol (Et 2 OH, 3.04g, 66mmol) and palladium chloride (PdCl 2 , 0.002g, 0.02mmol) of the mixture was dispersed in 80ml of benzene, the mixture was stirred at room temperature for 1h, and then the product A was obtained by distillation under reduced pressure.
[0057] (2) Characterization confirmation is the compound
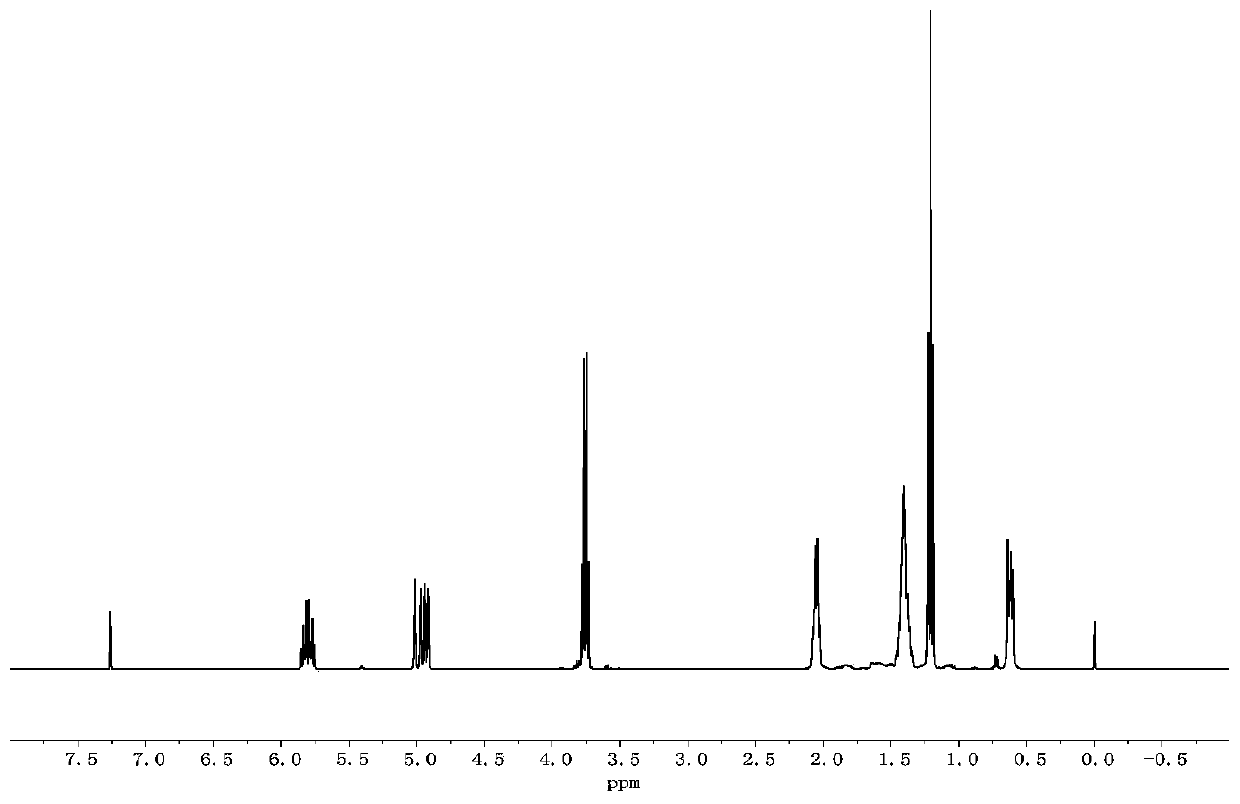
[0058] The hydrogen spectrum of A: 1 H NMR (300MHz, CDCl 3 ,ppm)δ:0.64(t,4H),1.22(t,6H),1.43(m,8H),2.05(m,4H),3.81(m,4H),4.94(q,4H),5.80(m ,2H). The hydrogen spectrum is as follows figure 2 shown.
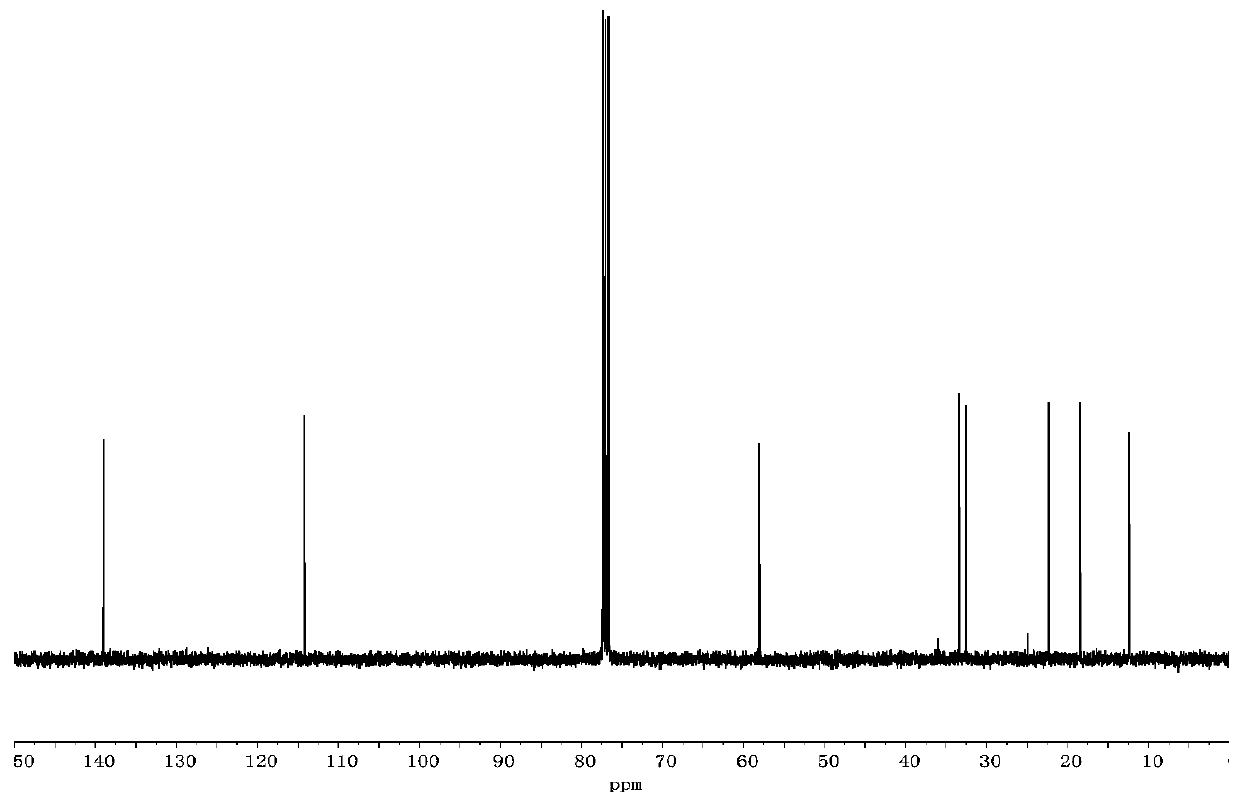
[0059] A carbon spectrum: 13 C NMR (300MHz, CDCl 3 , ppm) δ: 10.14, 32.21, 33.28 (CH 2 ), 18.16 (OCH 2 CH 3 ), 22.17 (SiCH 2 ), 58.18 (OCH 2 ), 114.06 (=CH 2 ), 138.87(CH). The carbon spectrum is as follows image 3 shown.
[0060] The silicon spectrum of A: 29 Si NMR(300MHz,ppm)δ:...
preparation example 2
[0063] (1) Preparation of dioctenyl dimethoxysilane
[0064] The steps are: under the protection of nitrogen, the dioctenylsilane (Octe 2 SiH 2 , 5.04g, 20mmol), absolute ethanol (MeOH, 3.26g, 102mmol) and palladium chloride (PdCl 2 , 0.004g, 0.04mmol) of the mixture was dispersed in 80ml of benzene, the mixture was stirred at room temperature for 1h, and then the product B was obtained by distillation under reduced pressure.
[0065] (2) Characterization confirmation is the compound
[0066] The hydrogen spectrum of B: 1 H NMR (300MHz, CDCl 3 ,ppm)δ:0.62(t,4H),1.35(m,16H),2.03(m,4H),3.56(m,6H),5.0(q,4H),5.78(m,2H). Figure such as Figure 5 shown.
[0067] Carbon spectrum of B: 13 C NMR (300MHz, CDCl 3 , ppm) δ: 11.86 (SiCH 2 ), 22.63, 28.75, 33.21, 33.80 (CH 2 ),50.26(OCH 3 ), 114.12 (=CH 2 ), 139.16(CH). The carbon spectrum is as follows Figure 6 shown.
[0068] Silicon spectrum of B: 29 Si NMR(300MHz,ppm)δ:-4.66(R 2 SiOCH 3 ).Silicon spectrum such as Fi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com