Method for purifying VEGF capture agent fusion protein
A purification method and fusion protein technology, applied in the field of purification of human vascular endothelial growth factor receptor-antibody fusion protein, can solve problems such as increasing the cost of drug production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
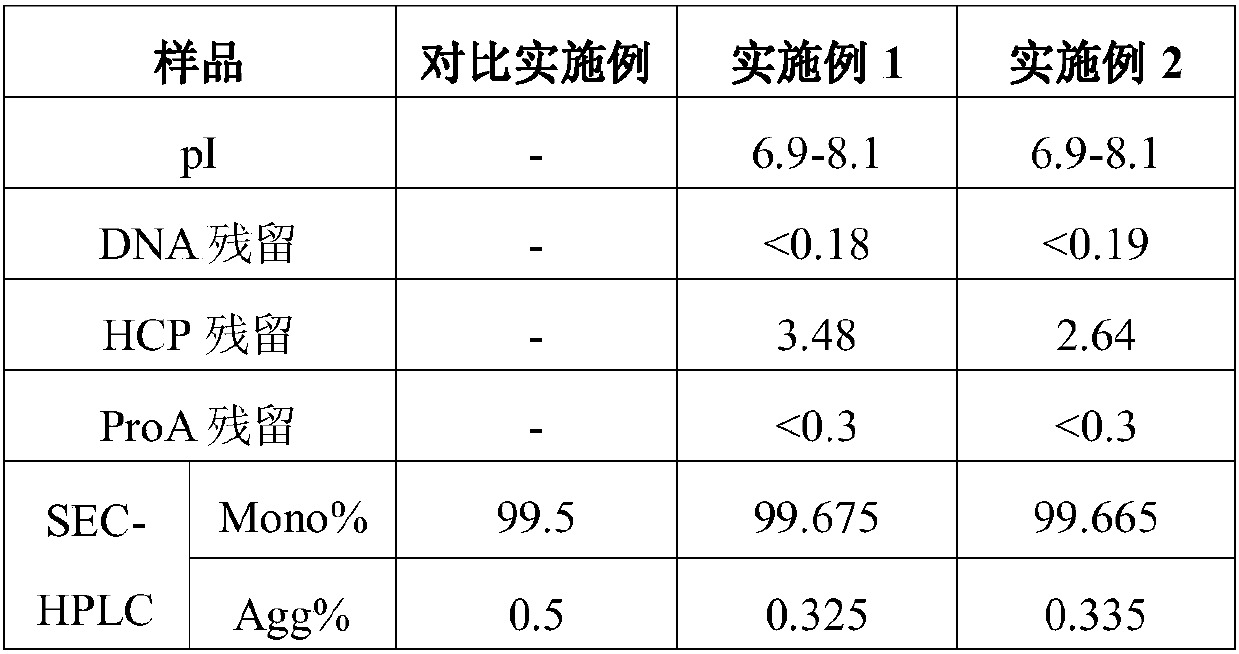
[0028] Take an appropriate amount of VEGF capture agent fusion protein fermentation broth, and purify according to the following steps and conditions, specifically including the following steps:
[0029] (1) Protein A affinity chromatography
[0030] Equilibrate buffer with 1L phosphate (pH7.0, 20mM NaH 2 PO 4 , 110mM NaCl) to equilibrate the affinity chromatography column MabSuReLX, and load the cell culture supernatant containing the VEGF capture agent fusion protein, so that the VEGF capture agent fusion protein is adsorbed on the protein A affinity medium. After loading the samples, the above equilibration buffer was used to wash, and then the protein was eluted with pH 2.90 and 100 mM Glycine buffer, and the eluate was collected. Then use 1mol / L phosphoric acid to acidify the eluate to pH 3.45 to inactivate the virus.
[0031] (2) Anion exchange chromatography
[0032] Equilibrate the anion-exchange chromatography column QFF with 1L Tris equilibrium buffer (pH8.40, 50...
Embodiment 2
[0037] Similar to Example 1, an appropriate amount of VEGF capture agent fusion protein fermentation broth was taken again, and purified according to the following steps and conditions, specifically as follows:
[0038] (1) Protein A affinity chromatography
[0039] Equilibrate buffer with phosphate (pH7.2, 20mM NaH 2 PO 4 , 110mM NaCl) to equilibrate the affinity chromatography column MabSuReLX, and load the cell culture supernatant containing the VEGF capture agent fusion protein encoded by SEQ ID NO:1, so that the VEGF capture agent is adsorbed on the protein A affinity medium . After loading the sample, wash with equilibration buffer, then use pH 3.00 Glycine (100 mM) eluent to elute the protein, and collect the eluate. The eluate was then acidified to pH 3.45 with phosphoric acid for virus inactivation.
[0040] (2) Anion exchange chromatography
[0041] Equilibrate the anion-exchange chromatography column QFF with Tris equilibrium buffer (pH8.50, 50mM Tris, 50mM NaC...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com