Pharmaceutical composition detecting method
A detection method and composition technology, which is applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve the problems of inability to accurately identify and quantify active ingredients, fail to detect active ingredient bellflower saponin D, and interfere with each other in qualitative and quantitative detection. , to achieve the effect of ensuring clinical efficacy, high recovery rate, and low background interference
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
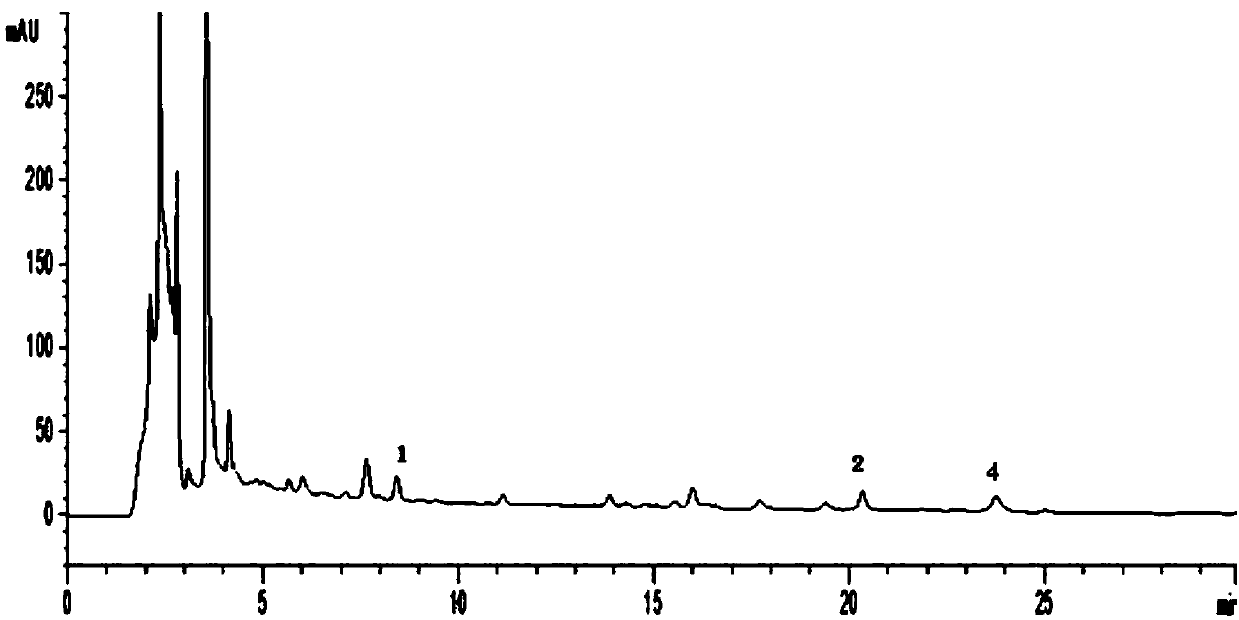
Examples
Embodiment 1
[0149] Scrophulariaceae extract, Radix Ophiopogon japonicus extract, Licorice root extract, Platycodon grandiflorum extract are prepared according to the following conventional extraction process:
[0150] Scrophulariaceae extract: Take Scrophulariaceae medicinal material, add water and decoct 3 times, 1 hour each time, combine the decoction, filter, let stand for 12 hours, concentrate the clear paste with a density of 1.10 (65-80°C), and dry it to obtain The extract powder is the Scrophulariaceae extract.
[0151] Extract of Ophiopogon japonicus: take Ophiopogon japonicus herb, add water to decoct 3 times, 1 hour each time, combine the decoction, filter, let stand for 12 hours, concentrate the clear paste with a density of 1.10 (65-80°C), and dry it to obtain The extract powder is the Ophiopogon japonicus extract.
[0152] Licorice extract: Take the licorice medicinal material, add water and decoct 3 times, 1 hour each time, combine the decoction, filter, let stand for 12 ho...
Embodiment 2
[0161] Need testing solution preparation and reference substance solution preparation are with embodiment 1;
[0162] Content detection: get each 10 μ l of reference substance solution and need testing solution and inject liquid chromatograph to detect, and its detection condition is as follows: with 0.05% phosphoric acid as mobile phase B, all the other detection conditions are with embodiment 1;
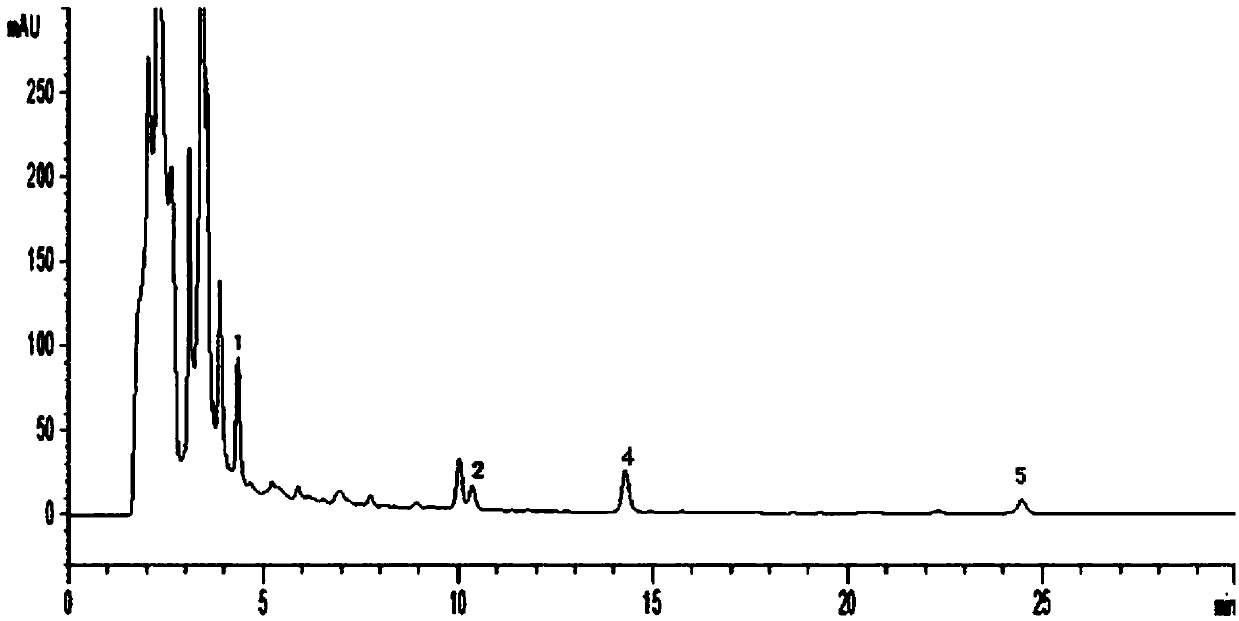
[0163] Carry out high-performance liquid chromatography detection according to above-mentioned method, the result is as follows Figure 9 shown. according to Figure 9 As shown, the retention time of the six index components is moderate, the resolution is greater than 1.5, the baseline drift value D is 5.0mAU / 30min, and the detection effect is achieved.
Embodiment 3
[0165] Need testing solution preparation and reference substance solution preparation are with embodiment 2;
[0166] Content detection: get each 10 μ l of reference substance solution and need testing solution and inject liquid chromatograph to detect, and its detection condition is as follows: with 0.2% phosphoric acid as mobile phase B, all the other detection conditions are the same as embodiment 1.
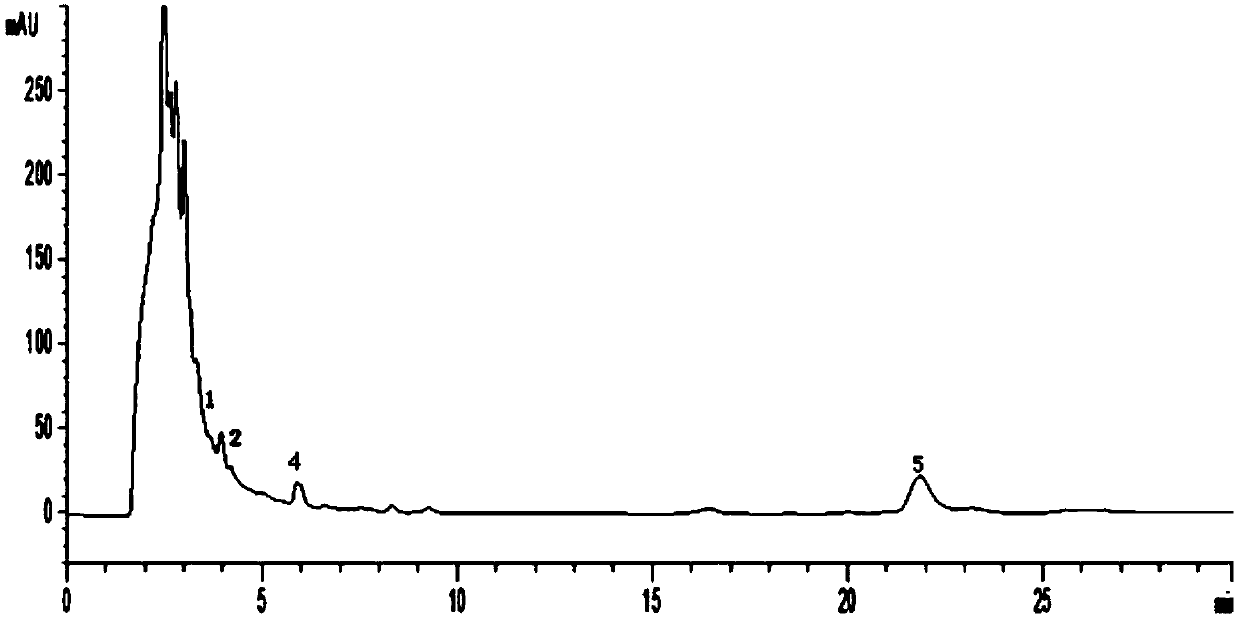
[0167] Carry out high-performance liquid chromatography detection according to above-mentioned method, the result is as follows Figure 10 shown. according to Figure 10 As shown, the retention time of the six index components is moderate, the resolution is greater than 1.5, and the baseline drift value D is 4.8mAU / 30min, achieving the detection effect.
PUM
| Property | Measurement | Unit |
|---|---|---|
| separation | aaaaa | aaaaa |
| separation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com