Application of tizoxanide and nitazoxanide in preparation of autophagy inducer
A technology of tizoxanide and nitazoxanide, which is applied in the direction of medical preparations containing active ingredients, organic active ingredients, drug combinations, etc., can solve the problems of cell autophagy that have not been reported in any way
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
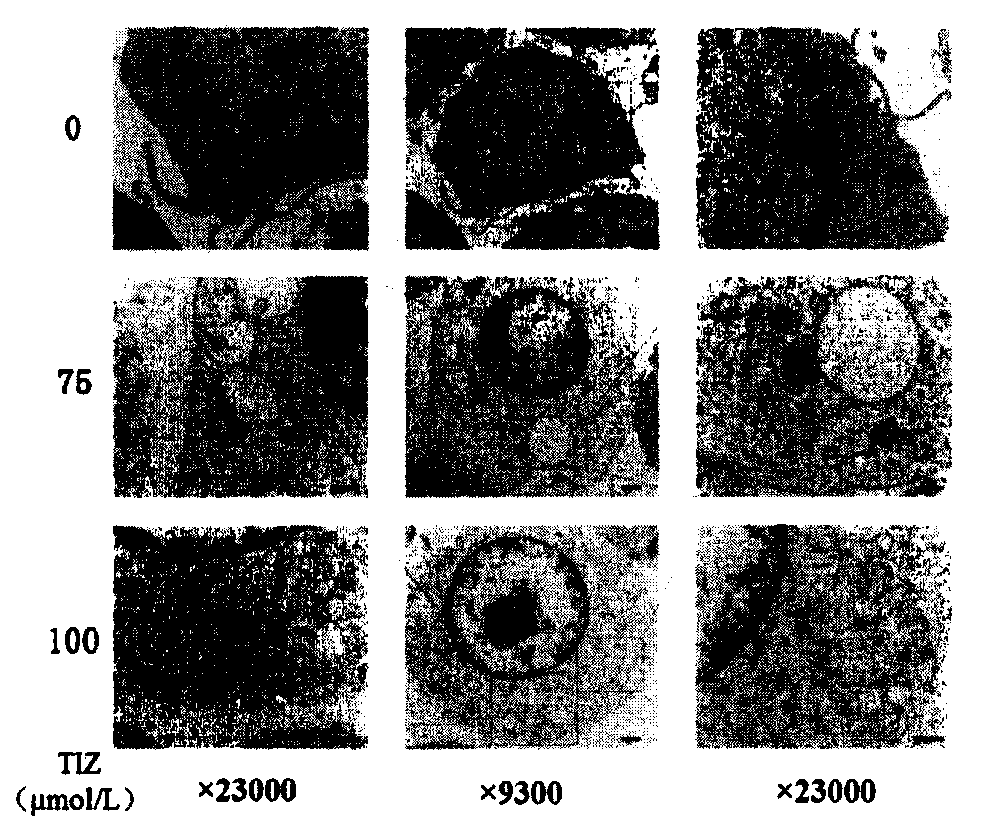
[0031] Induction of autophagy by tizoxanide in RAW264.7 cells detected by transmission electron microscope
[0032] 1. In vitro culture of RAW264.7 cell line
[0033]The cells used in this experiment were RAW264.7 cells, using DMEM medium supplemented with 10% fetal bovine serum (fetal bovine serum, FBS, Gibco), at 37°C, 5% CO 2 The cells were cultured in an incubator, and when the cells grew to 80-90% of the bottom of the culture dish, they were digested with 0.25% trypsin-EDTA and passaged.
[0034] 2. The effect of drugs on cell viability
[0035] Inoculate the well-grown cells in a 96-well plate at a density of 5×104 cells / mL, 100 μL per well, culture in a 37°C, 5% CO2 incubator for 12 hours, and then add different concentrations of tizoxanide or nitazoxanide , set 5 duplicate wells in each group, continue to cultivate for 12 hours, discard the original culture solution, add 10 μL of CCK-8 reagent to each well, continue to cultivate for 30 minutes, shake and mix well on ...
Embodiment 2
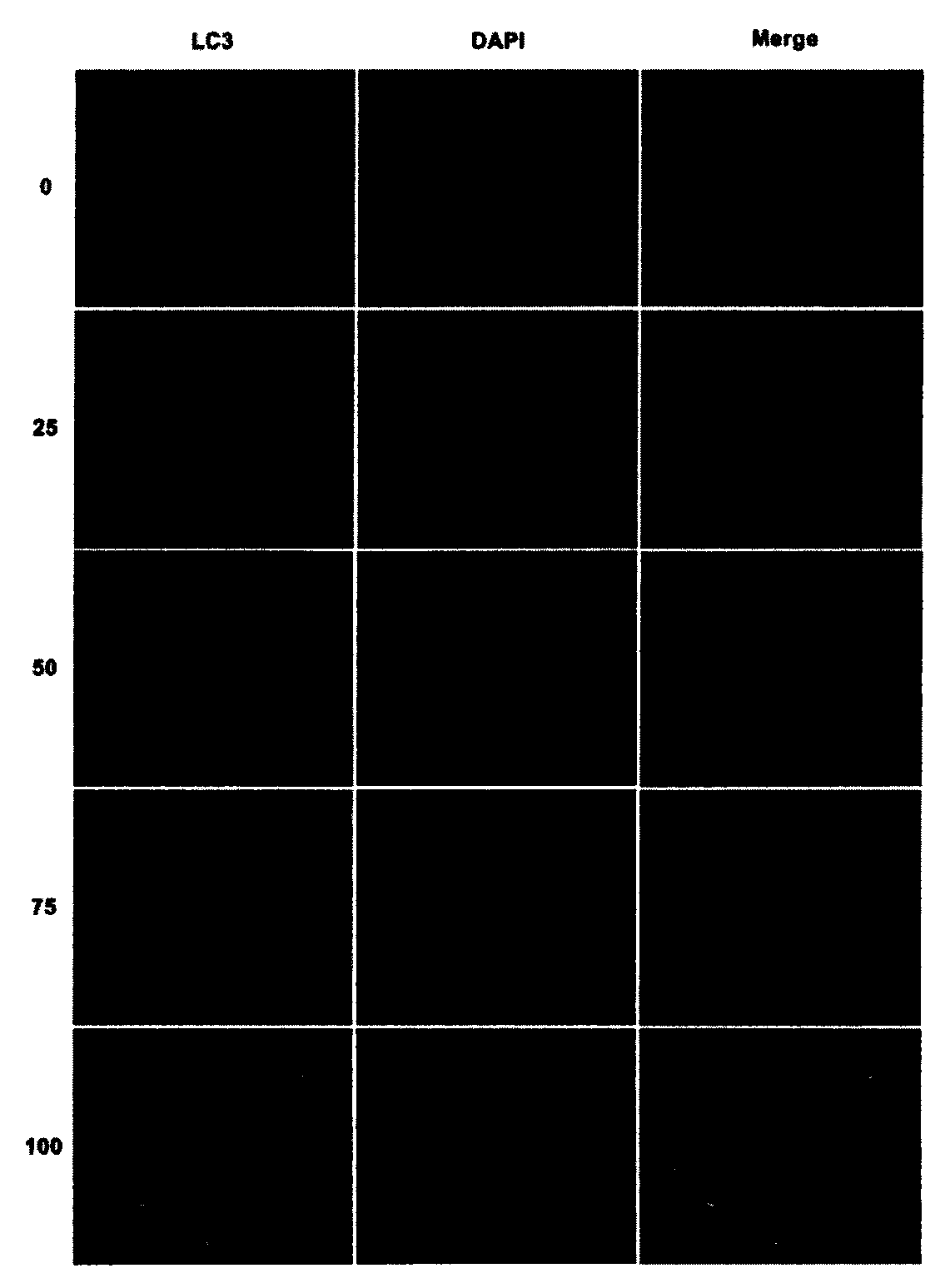
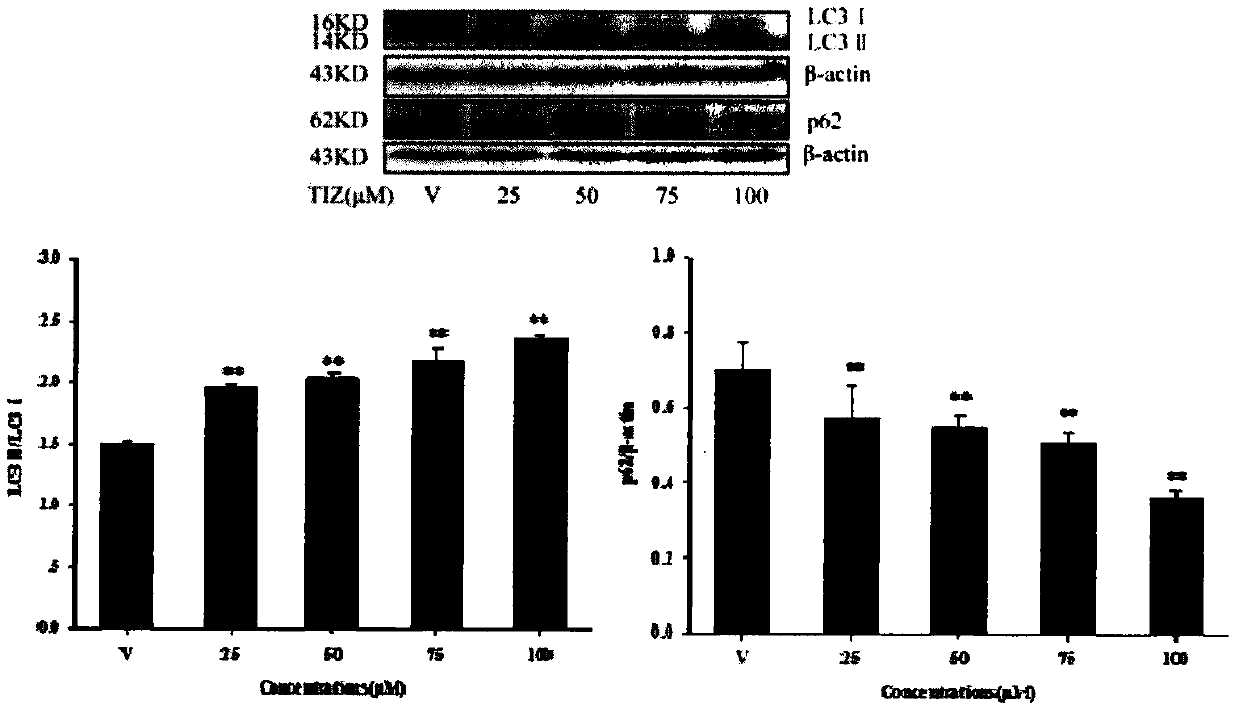
[0042] Detecting the induction effect of tizoxanide on cell autophagy by LC3 protein and p62 protein
[0043] LC3 is a landmark protein in the autophagy signaling pathway, which has two subtypes, LC3I and LC3II. When no autophagy occurs, the protein exists in the cytoplasm as a water-soluble LC3I isoform, and when cells undergo autophagy, the protein is converted into a water-insoluble LC3II isoform. By detecting the change of LC3II content in cells, the strength of autophagy in cells can be determined.
[0044] In addition, p62, also known as SQSTM1, is expressed in a variety of cells and tissues, and can be used as a truck protein to participate in various signal transduction processes. p62 can connect LC3 and ubiquitinated substrates, then be integrated into autophagosomes, and be degraded in autolysosomes; when autophagy is activated, autophagosomes fuse with lysosomes, autophagic vesicles Proteins or organelles such as p62 in vesicles are degraded by lysosomal enzymes, ...
Embodiment 3
[0055] Detecting the induction effect of nitazoxanide on autophagy by LC3 protein and p62 protein
[0056] LC3 is a landmark protein in the autophagy signaling pathway, which has two subtypes, LC3I and LC3II. When no autophagy occurs, the protein exists in the cytoplasm as a water-soluble LC3I isoform, and when cells undergo autophagy, the protein is converted into a water-insoluble LC3II isoform. By detecting the change of LC3II content in cells, the strength of autophagy in cells can be determined.
[0057] p62, also known as SQSTM1, is expressed in a variety of cells and tissues, and can be involved in various signal transduction processes as a truck protein. p62 can connect LC3 and ubiquitinated substrates, then be integrated into autophagosomes, and be degraded in autolysosomes; when autophagy is activated, autophagosomes fuse with lysosomes, autophagic vesicles Proteins or organelles such as p62 in vesicles are degraded by lysosomal enzymes, and p62 levels decrease; wh...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com