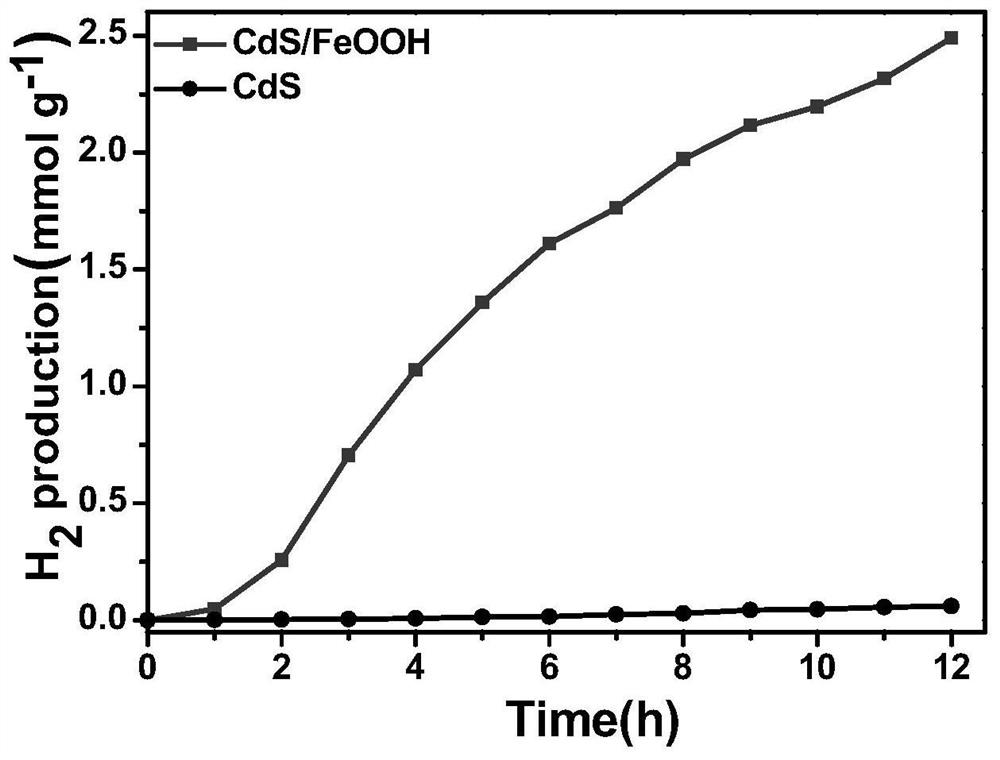
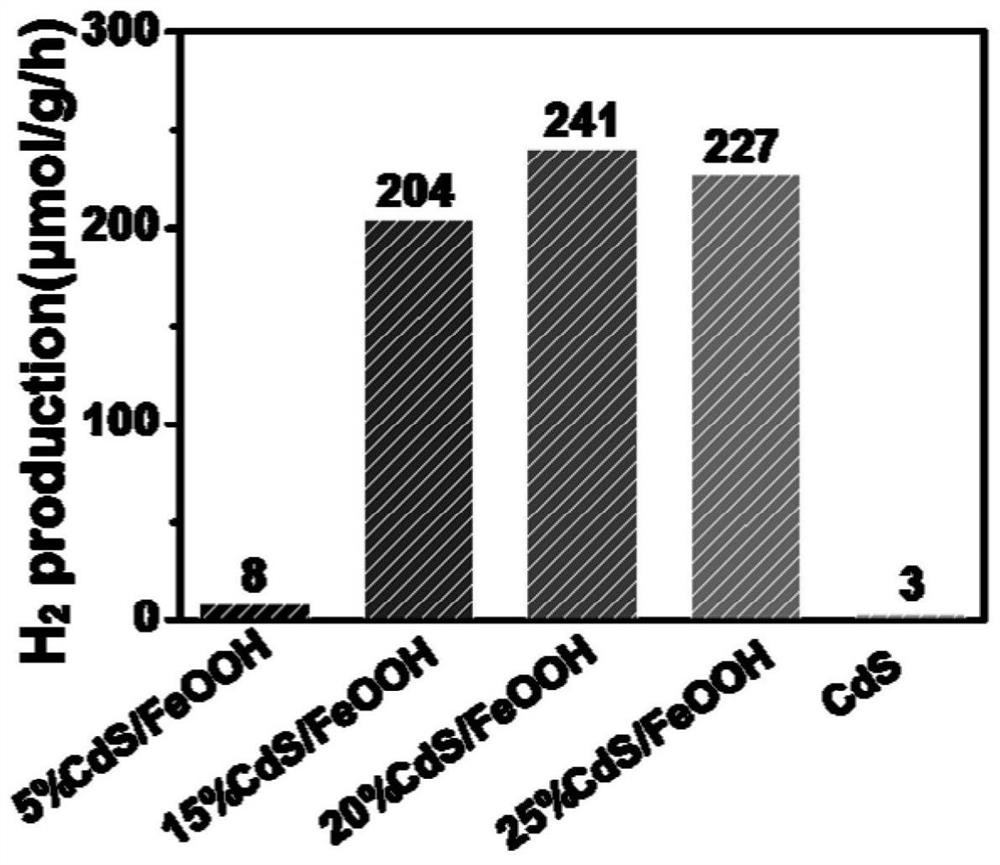
A kind of iron oxyhydroxide/cadmium sulfide nanobelt direct z-scheme photocatalyst and its preparation method
An iron oxyhydroxide, photocatalyst technology, applied in physical/chemical process catalysts, chemical instruments and methods, nanotechnology, etc., can solve the problem of different consumption rates of photogenerated electrons and holes, limit the efficiency of photocatalytic hydrogen production, and inhibit photogenerated electrons. Concentration and other issues, to achieve high redox potential, efficient photocatalytic water splitting to produce hydrogen, and improve photocatalytic efficiency.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] A preparation method of iron oxyhydroxide / cadmium sulfide nanobelt direct Z-scheme photocatalyst, the steps are as follows:
[0034] (1) 6.82mmol CdCl 2 2.5H 2 O and 13.64 mmol of sodium diethyldithiocarbamate were dissolved in 120 mL of ethylenediamine, and then placed at 180 ° C for 24 hours of heat preservation reaction, the solution after the reaction was filtered, washed with water and ethanol repeatedly, and finally the separated product was placed at 60 Dry at ℃ for 12 hours to obtain cadmium sulfide nanobelts;
[0035] (2) Weigh 0.1g of cadmium sulfide nanobelt and place it in 35ml of deionized water, stir it ultrasonically to make it evenly dispersed, then add 6.8g of sodium nitrate, stir for 10min, then add 0.009g of ferric chloride, stir for 10min, and finally wash with hydrochloric acid The pH of the solution was adjusted to 1.5-2, and then the stirred solution was placed in a 50ml hydrothermal reaction kettle and kept at 90°C for 4h. The reacted solution...
Embodiment 2
[0037] A preparation method of iron oxyhydroxide / cadmium sulfide nanobelt direct Z-scheme photocatalyst, the steps are as follows:
[0038] The preparation of cadmium sulfide nanobelts was carried out according to Example 1.
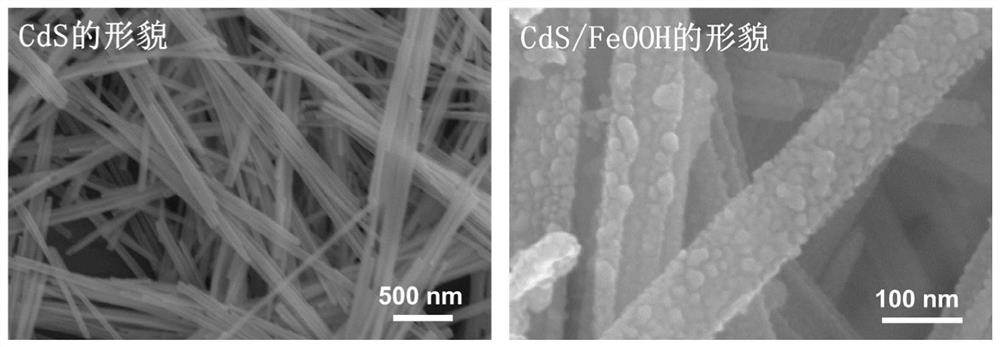
[0039] Weigh 0.1g of cadmium sulfide nanobelt and place it in 35ml of deionized water, stir it ultrasonically to make it evenly dispersed, then add 6.8g of sodium nitrate, stir for 10min, then add 0.036g of ferric chloride, stir for 10min, and finally adjust the pH of the solution with hydrochloric acid 1.5-2, then put the stirred solution in a 50ml hydrothermal reaction kettle, and keep it warm at 90°C for 4h. The reacted solution was filtered and washed repeatedly with ethanol and deionized water. Then, the separated product was kept at 60° C. for 12 hours to obtain the iron oxyhydroxide / cadmium sulfide nanobelt direct Z-scheme photocatalyst. The width of the cadmium sulfide nanobelt in the catalyst is 50-70nm, and the diameter of the iron oxyhydroxi...
Embodiment 3
[0045] A preparation method of iron oxyhydroxide / cadmium sulfide nanobelt direct Z-scheme photocatalyst, the steps are as follows:
[0046] The preparation of cadmium sulfide nanobelts was carried out according to Example 1.
[0047] Weigh 0.1g of cadmium sulfide nanobelt and place it in 35ml of deionized water, stir it ultrasonically to make it evenly dispersed, then add 6.8g of sodium nitrate, stir for 10min, then add 0.09g of ferric chloride, stir for 10min, and finally adjust the pH of the solution with hydrochloric acid 1.5-2, then put the stirred solution in a 50ml hydrothermal reaction kettle, and keep it warm at 90°C for 4h. The reacted solution was filtered and washed repeatedly with ethanol and deionized water. Then, the separated product was kept at 60° C. for 12 hours to obtain the iron oxyhydroxide / cadmium sulfide nanobelt direct Z-scheme photocatalyst. The width of the cadmium sulfide nanobelt in the catalyst is 50-70nm, and the diameter of the iron oxyhydroxid...
PUM
| Property | Measurement | Unit |
|---|---|---|
| width | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com