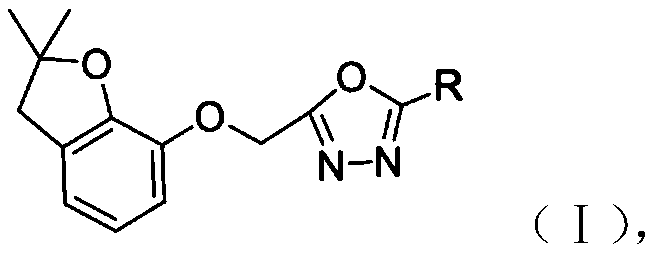
Benzofuranol-based oxadiazole derivative and preparation method and application thereof
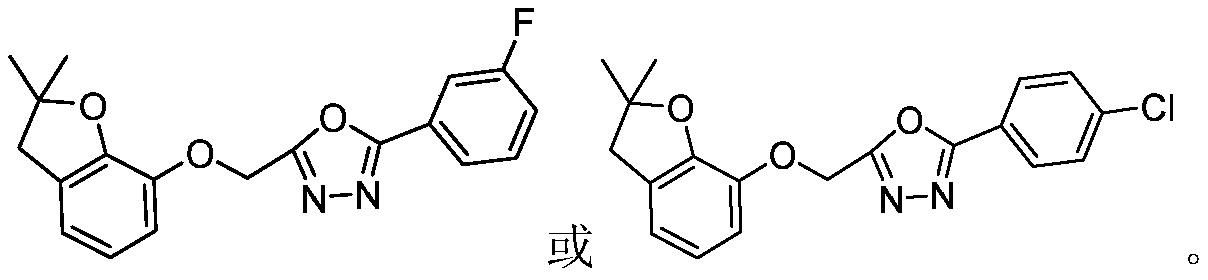
A compound and pharmaceutical technology, applied in the field of oxadiazole derivatives and their preparation, can solve the problems of low herbicidal activity, complicated preparation method and the like, and achieve the effects of simple preparation method, easy popularization and application, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
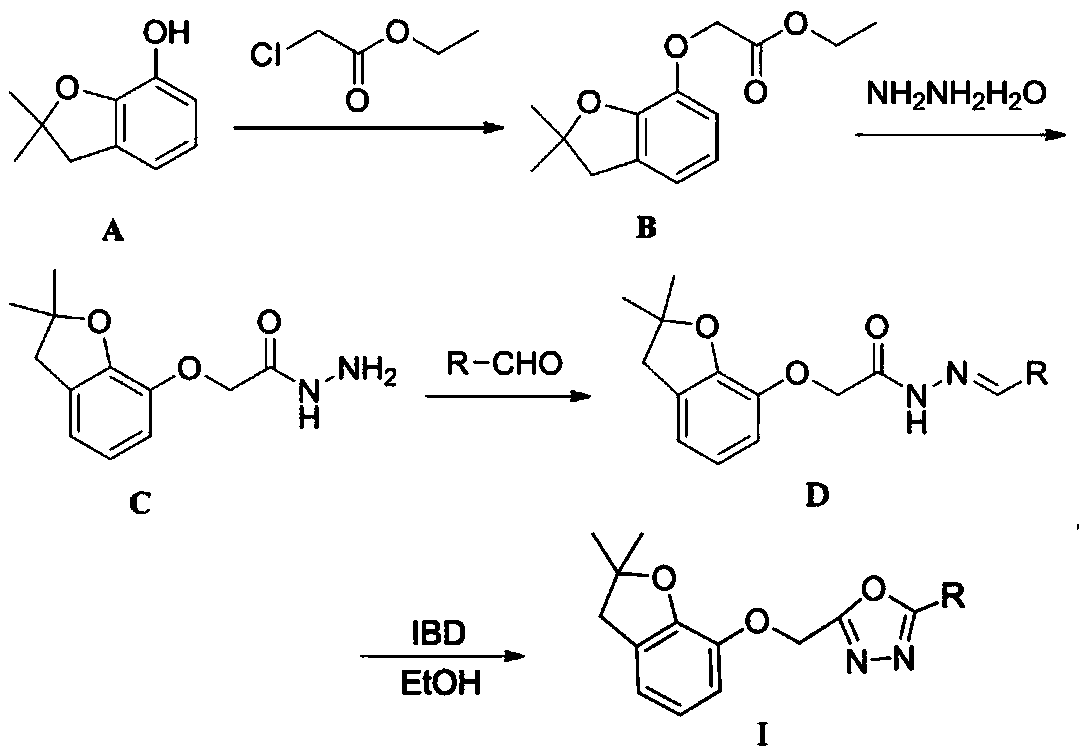
[0025] Synthesis of ethyl-2-((2,2-dimethyl-2,3-2,3-dihydrobenzofuran-7-yl)oxy)ethyl acetate (compound B):
[0026]
[0027] Dissolve compound A in anhydrous DMF, add K 2 CO 3 Stir at room temperature until dissolved, add ethyl chloroacetate, catalytic amount KI, stir and heat at 60°C for 6-8h, TLC detection. After the reaction, add a small amount of water and stir for 5 minutes, extract with ethyl acetate, and use anhydrous NaSO for the organic phase 4 To dry, spin dry. Petroleum ether: ethyl acetate = 40:1 column chromatography, the pure intermediate product B was obtained as a yellow viscous liquid with a yield of 90%. The molar ratio of compound A to ethyl chloroacetate is 1:1.5; MS-ESI, m / z 273.28[M+Na] + .HR-MS-EI m / z calcd for C 14 h 18 o 4 [M+H] + 250.2925, found 250.2940; 1 H NMR (600MHz, CDCl 3 )δ6.80(d,J=7.1Hz,1H),6.75–6.68(m,2H),4.71(s,2H),4.24(q,J=7.1Hz,2H),3.01(s,2H), 1.50(s,6H),1.28(t,J=7.1Hz,3H); 13 C NHR (150MHz, CDCl 3 )δ 169.34, 147.66, 142.65...
Embodiment 2
[0029] Synthesis of 2-((2,2-dimethyl-2,3-dihydrobenzofuran-7-yl)oxy)acetohydrazide (compound C):
[0030]
[0031] The intermediate product B was dissolved in absolute ethanol, and hydrazine hydrate (80% mass fraction) was added dropwise under ice-bath conditions, and stirred for 5 min under ice-bath conditions. Stir and heat at 60°C for 2h, monitored by TLC. After the reaction, dichloromethane was extracted, the solvent was spin-dried, and water was added, crystals were precipitated, and the mixture was suction-filtered. Recrystallization from ethanol gave intermediate product C as white crystals with a yield of 93%. The molar ratio of intermediate product B to hydrazine hydrate is 1:2.5; MS-ESI, m / z 259.26[M+Na] + .HR-MS-EI m / z calcd for C 12 h 16 N 2 o 3 [M+H] + 236.2695,found 236.2710; 1 H NMR (600MHz, CDCl 3 )δ8.13(s,1H),6.86(d,J=7.2Hz,1H),6.77(t,J=7.7Hz,1H),6.73(d,J=8.0Hz,1H),4.65(s, 2H), 3.90(s,2H), 3.05(s,2H), 1.52(s,6H).
Embodiment 3
[0033]Preparation of (E)-2-((2,2-dimethyl-2,3-dihydrobenzofuran-7-yl)oxy)-N'-(4-fluorobenzylidene)acetylhydrazide :
[0034]
[0035] 2.4mmol of 2-((2,2-dimethyl-2,3-dihydrobenzofuran-7-yl)oxy)acetohydrazide was dissolved in 50ml of ethanol, stirred and dissolved, then added 2.88mmol of p-fluorobenzaldehyde, oil Heat and stir the bath at 60°C for 6-8h, monitor by TLC. After the reaction is complete, filter with suction and spin-dry to obtain 0.74g of the crude product of light yellow viscous solid, which is subjected to column chromatography (V 二氯甲烷 :V 甲醇 =160:1), to obtain (E)-2-((2,2-dimethyl-2,3-dihydrobenzofuran-7-yl)oxy)-N'-(4-fluorophenylene Methyl) acetylhydrazide 0.54g. m.p.145.9-146.5℃, yield 90.1%, 1 H NMR (600MHz, CDCl 3 )δ9.92(s,1H),8.13(s,1H),7.63(d,J=8.4Hz,2H),7.30(d,J=8.4Hz,2H),6.84–6.79(m,1H), 6.73 (d, J=6.3Hz, 2H), 4.67 (s, 2H), 2.99 (s, 2H), 1.46 (s, 6H).
[0036] Preparation of 2-(((2,2-dimethyl-2,3-dihydrobenzofuran-7-yl)oxy)methyl)-5-(4-fluorophe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com