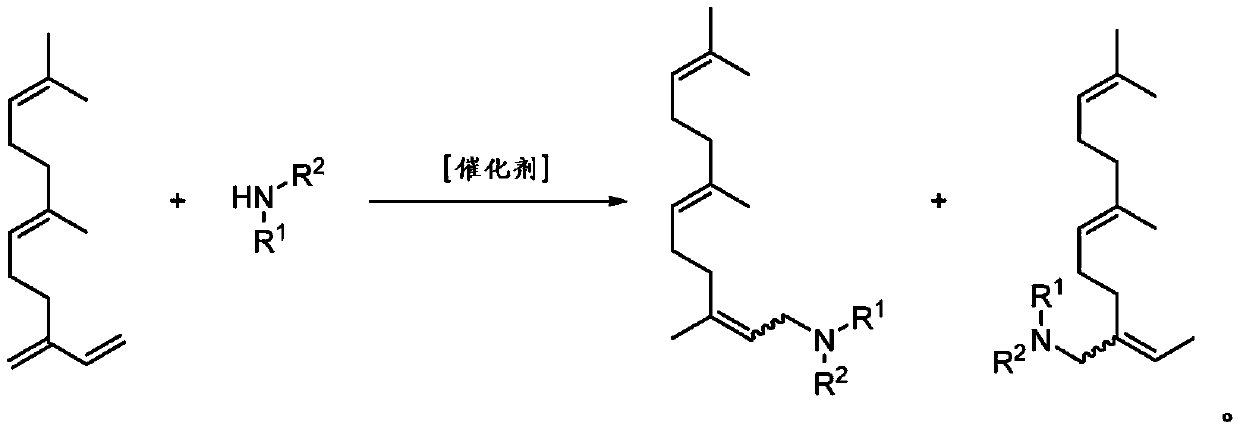
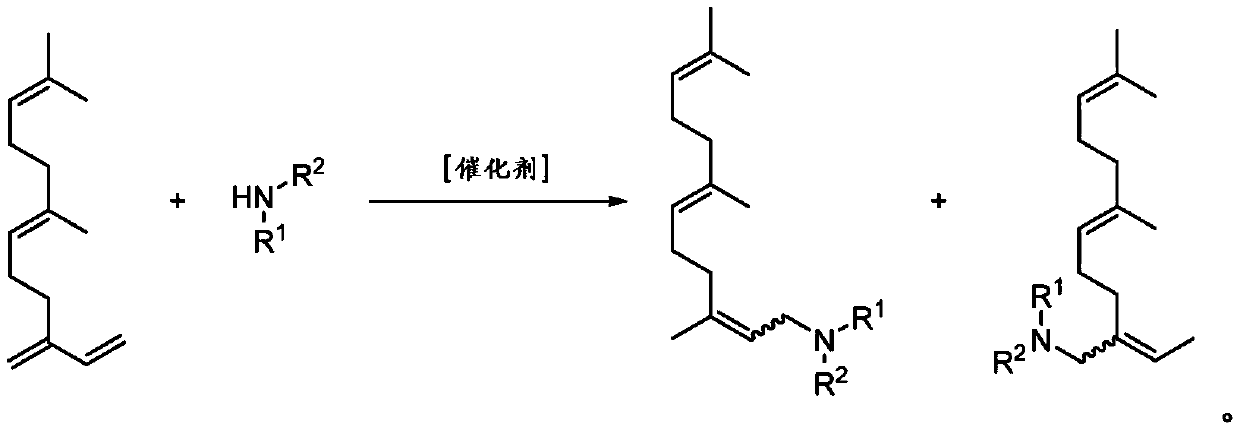
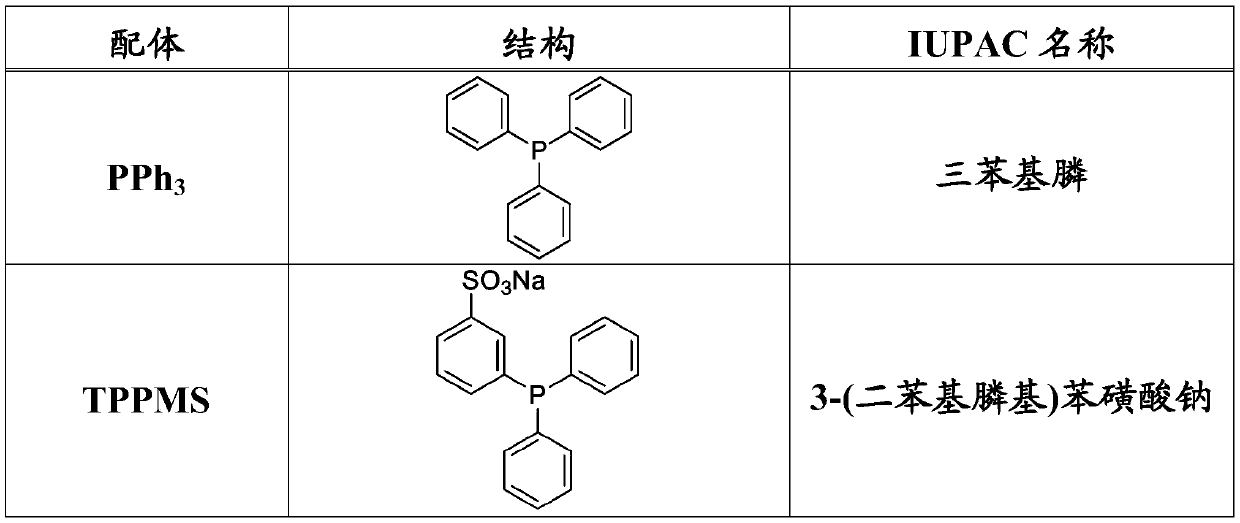
Amine derivatives of the beta-farnesene
A technology of farnesene and farnesamide, which is applied in the field of amine derivatives and can solve problems such as ineffective reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] Weigh 16.6 mg of Pd(hfacac) in a 25 mL steel autoclave 2 and 109.3 mg of DPPB and dissolved in 5 mL of DMF. Then 817.4 mg of β-farnesene and 296.9 mg of diethylamine were added. The reactor was closed and loaded with argon pressure of 5 bar.
[0070] The reactor was heated at 100 °C for 5 h and stirred at 500 rpm by means of a magnetic stirrer. To terminate the reaction, the reactor was cooled to room temperature and then argon was carefully vented.
[0071] The obtained reaction solution was freed of solvent under vacuum and the product was purified by column chromatography. 863.2 mg (78%) of the hydroaminated product were obtained.
Embodiment 2
[0073] Weigh 10.6 mg of Pd(tfa) in a 25 mL steel autoclave 2 and 137.8 mg of DPEPhos and dissolved in 5 mL of methanol. Then 817.7 mg of β-farnesene and 297.1 mg of diethylamine were added. The reactor was closed and loaded with argon pressure of 5 bar.
[0074] The reactor was heated at 100 °C for 5 h and stirred at 500 rpm by means of a magnetic stirrer. To terminate the reaction, the reactor was cooled to room temperature and then argon was carefully vented.
[0075] The obtained reaction solution was freed of solvent under vacuum and the product was purified by column chromatography. 960.4 mg (89%) of the hydroaminated product were obtained.
Embodiment 3
[0077] Weigh 10.5 mg of Pd(tfa) in a 25 mL steel autoclave 2 and 137.7 mg of DPEPhos and dissolved in 5 mL of anisole. Then 816.8 mg of β-farnesene and 516.6 mg of dibutylamine were added. The reactor was closed and loaded with argon pressure of 5 bar.
[0078] The reactor was heated at 100 °C for 5 h and stirred at 500 rpm by means of a magnetic stirrer. To terminate the reaction, the reactor was cooled to room temperature and then argon was carefully vented.
[0079] The obtained reaction solution was freed of solvent under vacuum and the product was purified by column chromatography. 960.8 mg (72%) of the hydroaminated product were obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com