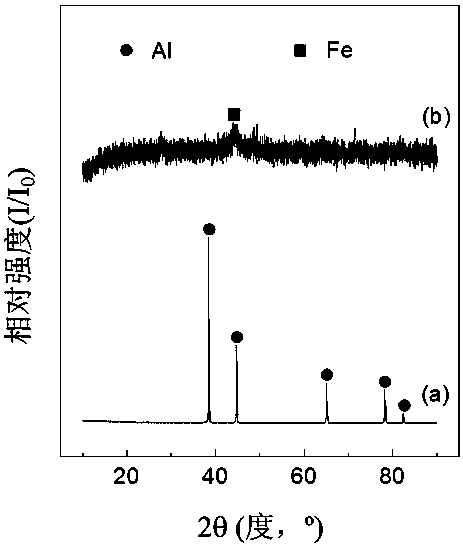
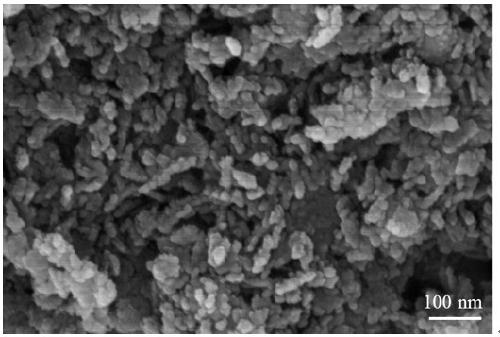
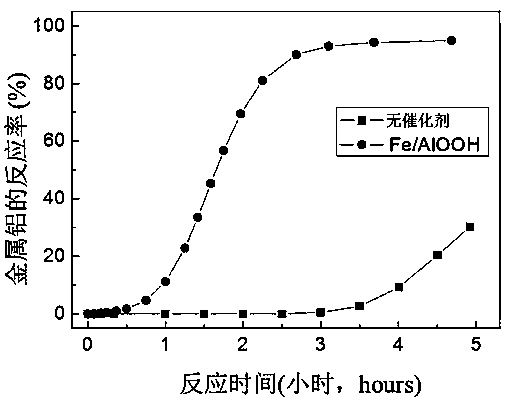
Preparation method and application of Fe/AlOOH catalyst
A catalyst, fecl3 6h2o technology, applied in physical/chemical process catalysts, chemical instruments and methods, metal/metal oxide/metal hydroxide catalysts, etc., can solve the complex preparation process, difficult reaction control, and corrosiveness and other problems, to achieve the effect of simple operation, simple hydrogen production process and no pollution to the environment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Example Embodiment
[0026] Example 1
[0027] The preparation method of Fe / AlOOH catalyst includes the following steps:
[0028] a. At room temperature (25℃), ultrasonic power is 150W, ultrasonic frequency is 40kHz, add AlOOH powder into a beaker with deionized water and place in an ultrasonic bath for ultrasonic mixing for 1 hour, then add FeCl 3 ·6H 2 O, continue to ultrasonically mix for 0.5 h to obtain suspension A, the mass ratio of AlOOH powder to water is 1:250, AlOOH powder and FeCl 3 ·6H 2 The molar ratio of O is 1:1;
[0029] b. Add the suspension A in step a to the sodium borohydride within 30 seconds, mix (stir manually), and react until a black precipitate is formed, without bubbles, to obtain the Fe / AlOOH suspension, sodium borohydride and FeCl 3 ·6H 2 The molar ratio of O is 3:1;
[0030] c. Suction and filter the Fe / AlOOH suspension in step b, first wash with deionized water 3 times, then wash with absolute ethanol once, filter, and dry at 25°C for 10 h to obtain Fe / AlOOH catalyst .
[003...
Example Embodiment
[0033] Example 2
[0034] The preparation method of Fe / AlOOH catalyst includes the following steps:
[0035] a. At room temperature (25℃), ultrasonic power is 120W, ultrasonic frequency is 30kHz, add AlOOH powder into a beaker with deionized water, place it in an ultrasonic bath for ultrasonic mixing for 3 hours, add FeCl 3 ·6H 2 O, continue to ultrasonically mix for 2 h to obtain suspension A, the mass ratio of AlOOH powder to water is 1:500, AlOOH powder and FeCl 3 ·6H 2 The molar ratio of O is 2:1;
[0036] b. Add the suspension A in step a to the sodium borohydride within 30 seconds, mix (stir manually), and react until a black precipitate is formed, without bubbles, to obtain the Fe / AlOOH suspension, sodium borohydride and FeCl 3 ·6H 2 The molar ratio of O is 3:1;
[0037] c. Suction and filter the Fe / AlOOH suspension in step b, first wash with deionized water 3 times, then wash with absolute ethanol once, filter, and dry at 25°C for 10 h to obtain Fe / AlOOH catalyst .
[0038] Usi...
Example Embodiment
[0040] Example 3
[0041] The preparation method of Fe / AlOOH catalyst includes the following steps:
[0042] a. At room temperature (25℃), ultrasonic power is 300W, ultrasonic frequency is 50kHz, add AlOOH powder into a beaker with deionized water, place it in an ultrasonic water bath and ultrasonically mix for 0.5h, then add FeCl 3 ·6H 2 O, continue to ultrasonically mix for 0.5h to obtain suspension A, the mass ratio of AlOOH powder to water is 1:100, AlOOH powder and FeCl 3 ·6H 2 The molar ratio of O is 0.5:1;
[0043] b. Add the suspension A in step a to the sodium borohydride within 30 seconds, mix (stir manually), and react until a black precipitate is formed, without bubbles, to obtain the Fe / AlOOH suspension, sodium borohydride and FeCl 3 ·6H 2 The molar ratio of O is 3:1;
[0044] c. Suction and filter the Fe / AlOOH suspension in step b, first wash with deionized water 3 times, then wash with absolute ethanol once, filter, and dry at 25°C for 10 h to obtain Fe / AlOOH catalyst ....
PUM
| Property | Measurement | Unit |
|---|---|---|
| Average size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap