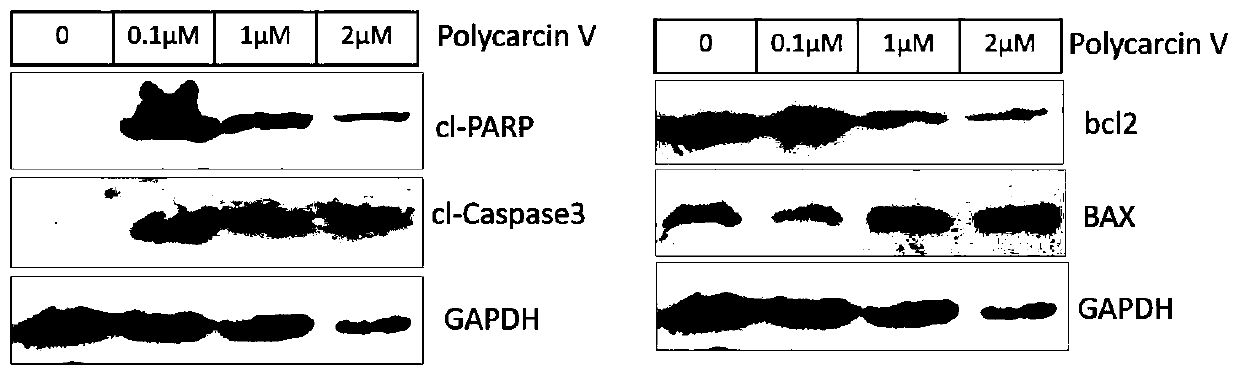
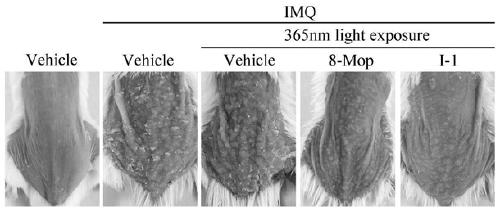
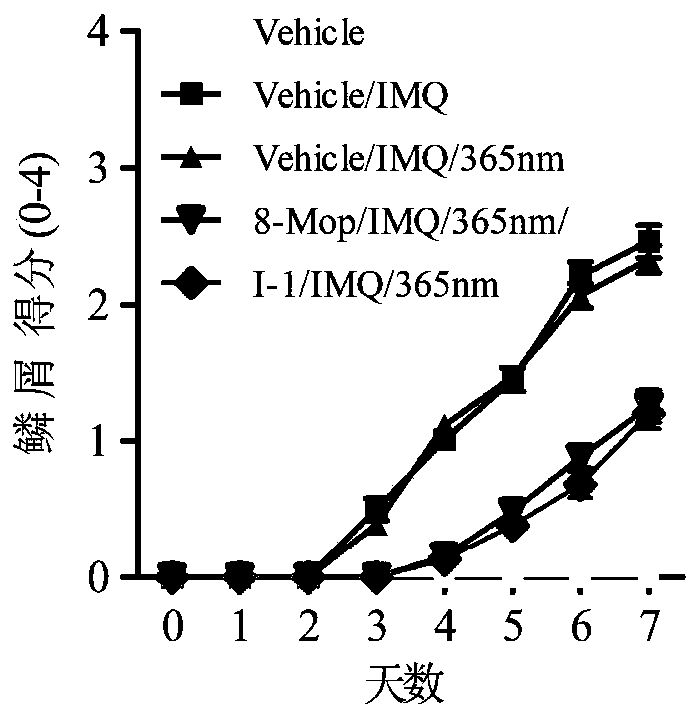
Compound and synthesis method and application thereof
A technology of compound and glucose, which is applied in the field of preparation of drugs, can solve problems such as poor effect and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0184] Embodiment 1: polycarcin V and its synthesis
[0185] Follow the steps below to synthesize polycarcin V:
[0186]
[0187] (1) Synthesis of compound 2:
[0188] Compound 1 (2.34 g, 9.24 mmol) was dissolved in 72 mL of anhydrous N,N-dimethylformamide at room temperature. Then the solution was cooled to 0° C. by ice-water bath, and sodium hydride (60% in mineral oil, 665 mg, 16.63 mmol) was added slowly and carefully. The resulting mixture was stirred for 30 minutes, then isopropyl iodide (1.88 mL, 18.48 mmol) was added via syringe. The reaction solution was heated to 70 °C and stirred overnight, then cooled to room temperature. The reaction solution was quenched by pouring into ice-cold water, and extracted with dichloromethane. The combined organic phases were dried over anhydrous sodium sulfate, filtered, and concentrated under reduced pressure to obtain a crude product. Separation and purification by silica gel column chromatography (petroleum ether / ethyl acetat...
Embodiment 2
[0220] Embodiment 2: Compound I-4 and its synthesis
[0221] Derivative 1-4 was synthesized according to the following steps:
[0222]
[0223] (1) Synthesis of Compound 23:
[0224] To a solution of aromatic ring 8 (43.7 mg, 0.125 mmol) and sugar donor 22 (49.8 mg, 0.105 mmol) in anhydrous dichloroethane (0.6 mL) was added at room temperature Molecular sieves (1.00g), tin tetrachloride (1.0M dichloroethane solution, 0.31mL, 0.305mmol). The reaction mixture was stirred at room temperature for 18 hours, then dichloromethane and saturated sodium bicarbonate solution were added. After separation of the organic phase, it was washed with water and then concentrated under reduced pressure. The residue was separated and purified by preparative thin layer chromatography (CH 2 Cl 2 / EtOAc=98 / 2) gave compound 8 (29.7 mg, 37%) as a yellow solid. Its characterization information is specifically:
[0225] 1 H NMR (400MHz, CDCl3) δ9.71(s, 1H), 8.22(s, 1H), 8.11–8.01(m, 2H), 7.75...
Embodiment 3
[0229] Embodiment 3: Compound 1-5 and its synthesis
[0230] Derivative 1-5 was synthesized according to the following steps:
[0231]
[0232] (1) Synthesis of Compound 25:
[0233]To a solution of aromatic ring 8 (400 mg, 1.204 mmol) and sugar donor 24 (105.0 mg, 0.301 mmol) in anhydrous dichloroethane (17.7 mL) was added tin tetrachloride (1.0 M dichloroethane solution, 2.7mL, 2.709mmol). The reaction mixture was stirred at room temperature for 46 hours, then dichloromethane and saturated sodium bicarbonate solution were added. After separation of the organic phase, it was washed with water and then concentrated under reduced pressure. The residue was separated and purified by preparative thin layer chromatography (CH 2 Cl 2 / EtOAc=98 / 2) gave compound 25 (15.1 mg, 8%) as a yellow solid. Its characterization information is specifically:
[0234] 1 H NMR (400MHz, CDCl3) δ9.52(s, 1H), 8.01–7.95(m, 3H), 7.68(d, J=8.8Hz, 1H), 7.15(s, 1H), 6.68(dd, J= 17.5,10.9Hz,1H),...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com