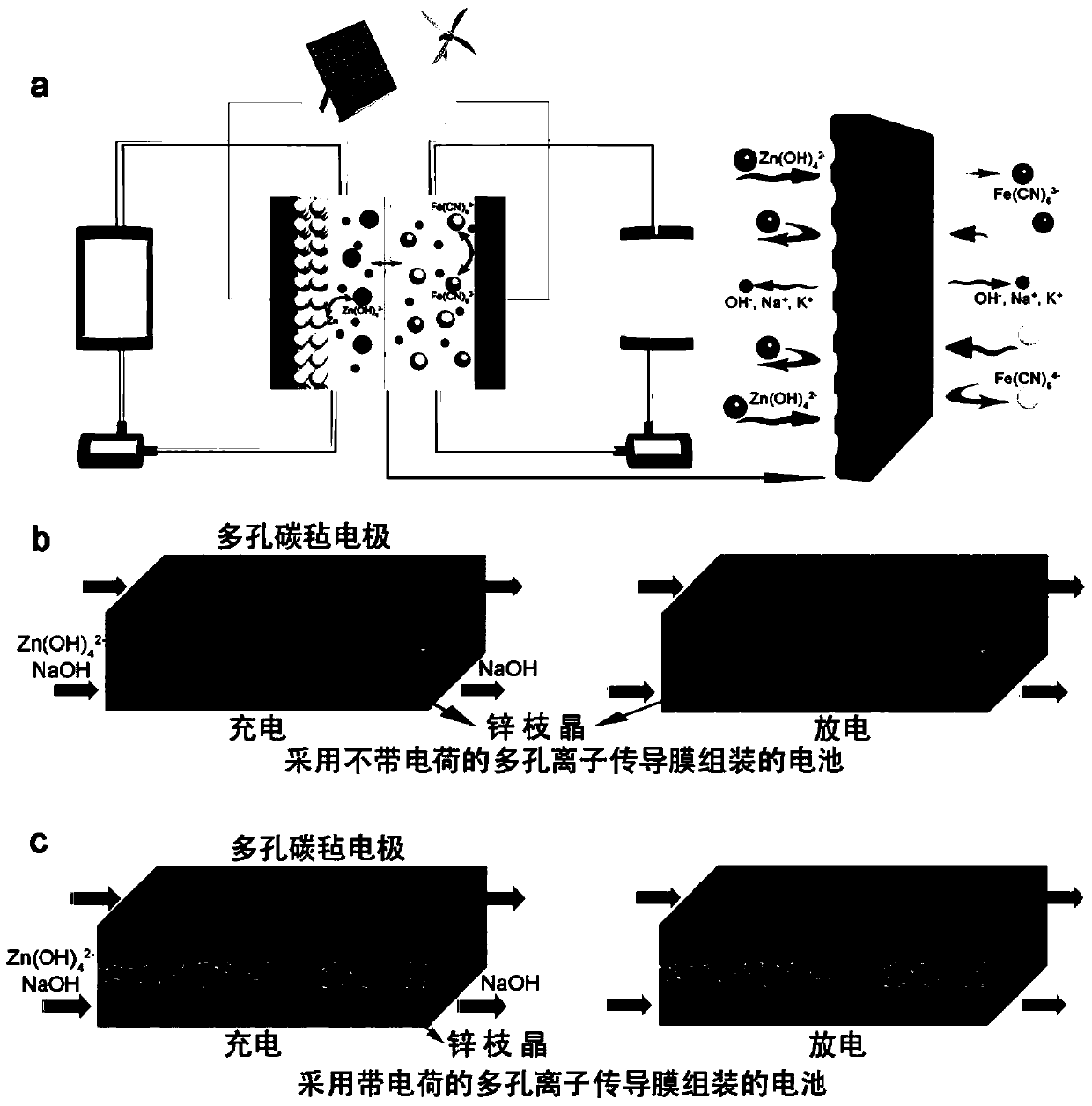
Application of a Porous Ion Conducting Membrane with Negatively Charged Membrane Surface in Alkaline Zinc-Based Batteries
An ion-conducting membrane, negative charge technology, applied in battery pack components, separators/films/diaphragms/spacers, circuits, etc., can solve problems such as battery short circuits, and achieve the effect of alleviating damage and improving zinc accumulation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
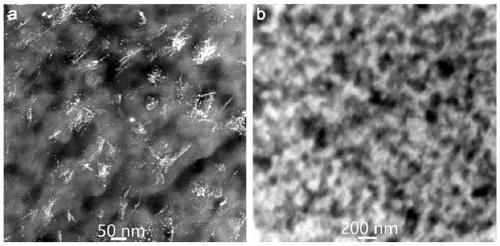
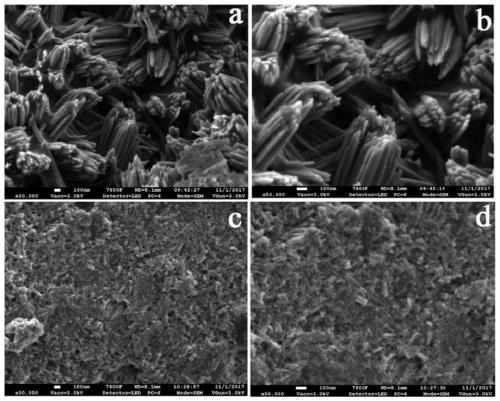
[0048] Using PES / PVP as the base material, dissolve PES / PVP in DMAC solvent to obtain a blend solution with a mass concentration of 35%, wherein the mass ratio of PES to PVP is 1:1, pour the above blend solution on a clean and flat glass On the board, the solvent was volatilized for 10 s under the condition of 20% humidity, and then the whole was immersed in water for 700 s, and a porous ion-conducting membrane was prepared at 25 ° C. After removing PVP, an uncharged PES porous ion-conducting membrane (pore size range : 0.9~40 nm, porosity: ~67%). The above-mentioned uncharged porous membrane was soaked in a solution containing 10wt% benzophenone (BP) for 30min, then its surface was wiped dry and transferred to a solution of 8wt% sodium p-styrene sulfonate. Under the following conditions, a UV light source with a dominant wavelength of 380 nm was used for grafting for 120 minutes to obtain a porous ion-conducting membrane (PES-g-PSNa) with negative charges on one side. The uni...
Embodiment 2
[0052] Using PES / PVP as the base material, dissolve PES / PVP in DMAC solvent to obtain a blend solution with a mass concentration of 35%, wherein the mass ratio of PES to PVP is 1:1, pour the above blend solution on a clean and flat glass On the board, the solvent was volatilized for 10 s under the condition of 20% humidity, and then the whole was immersed in water for 700 s, and a porous ion-conducting membrane was prepared at 25 ° C. After removing PVP, an uncharged PES porous ion-conducting membrane (pore size range : 0.9~40 nm, porosity: ~67%). The above-mentioned uncharged porous membrane was soaked in a solution containing 10wt% benzophenone (BP) for 30min, then its surface was wiped dry and transferred to a solution of 8wt% sodium p-styrene sulfonate. Under the following conditions, a UV light source with a dominant wavelength of 380 nm was used to irradiate grafting for 70 minutes to obtain a porous ion-conducting membrane (PES-g-PSNa) with negative charges on one side,...
Embodiment 3
[0056] Using PES / PVP as the base material, dissolve PES / PVP in DMAC solvent to obtain a blend solution with a mass concentration of 35%, wherein the mass ratio of PES to PVP is 1:1, pour the above blend solution on a clean and flat glass On the board, the solvent was volatilized for 10 s under the condition of 20% humidity, and then the whole was immersed in water for 700 s, and a porous ion-conducting membrane was prepared at 25 ° C. After removing PVP, an uncharged PES porous ion-conducting membrane (pore size range : 0.9~40 nm, porosity: ~67%). The above-mentioned uncharged porous membrane was soaked in a solution containing 10wt% benzophenone (BP) for 30min, then its surface was wiped dry and transferred to a solution of 8wt% sodium p-styrene sulfonate. Under the following conditions, a UV light source with a dominant wavelength of 380 nm was used for grafting for 70 minutes to obtain a porous ion-conducting membrane (PES-g-PSNa) with negative charges on one side, which wa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com