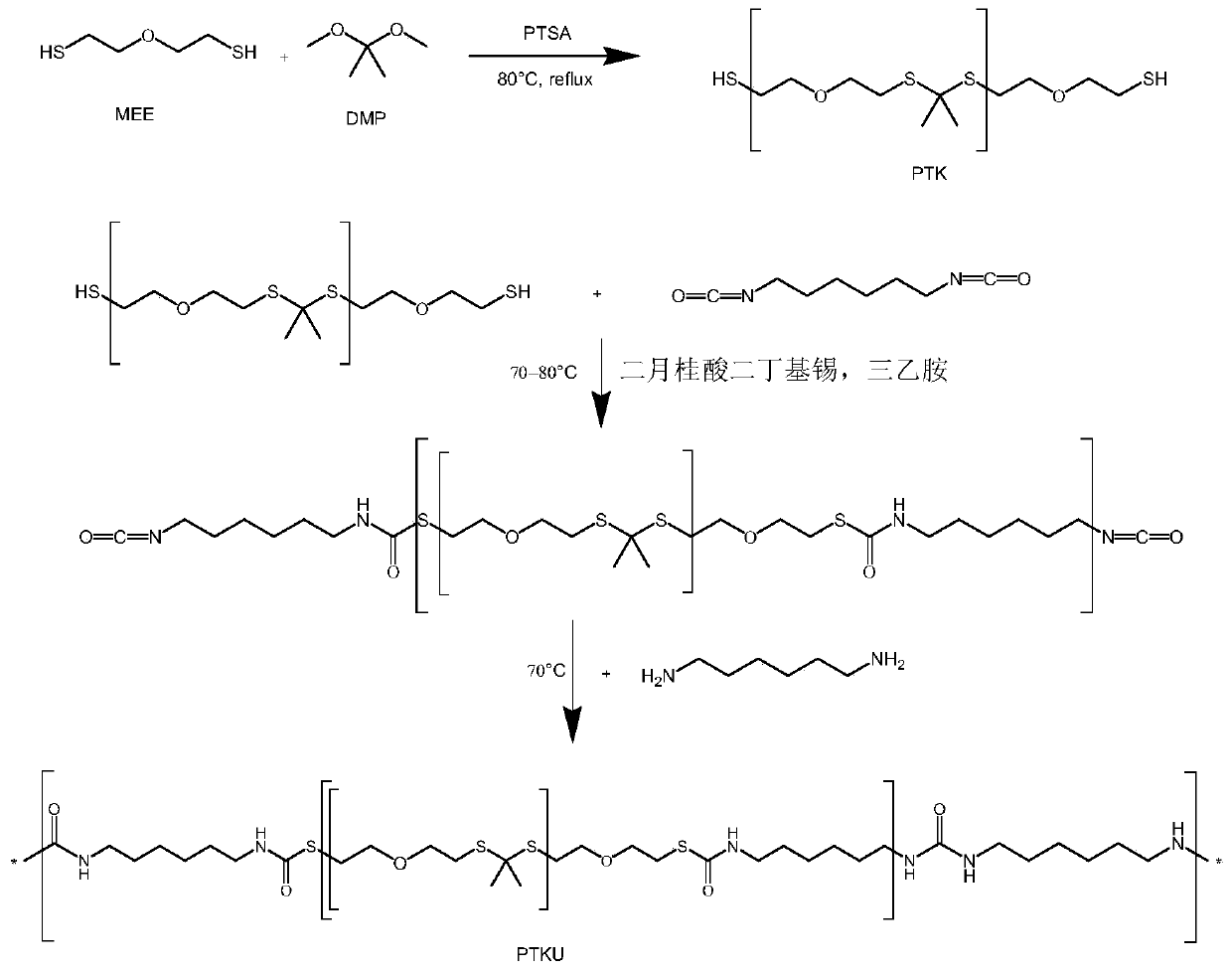
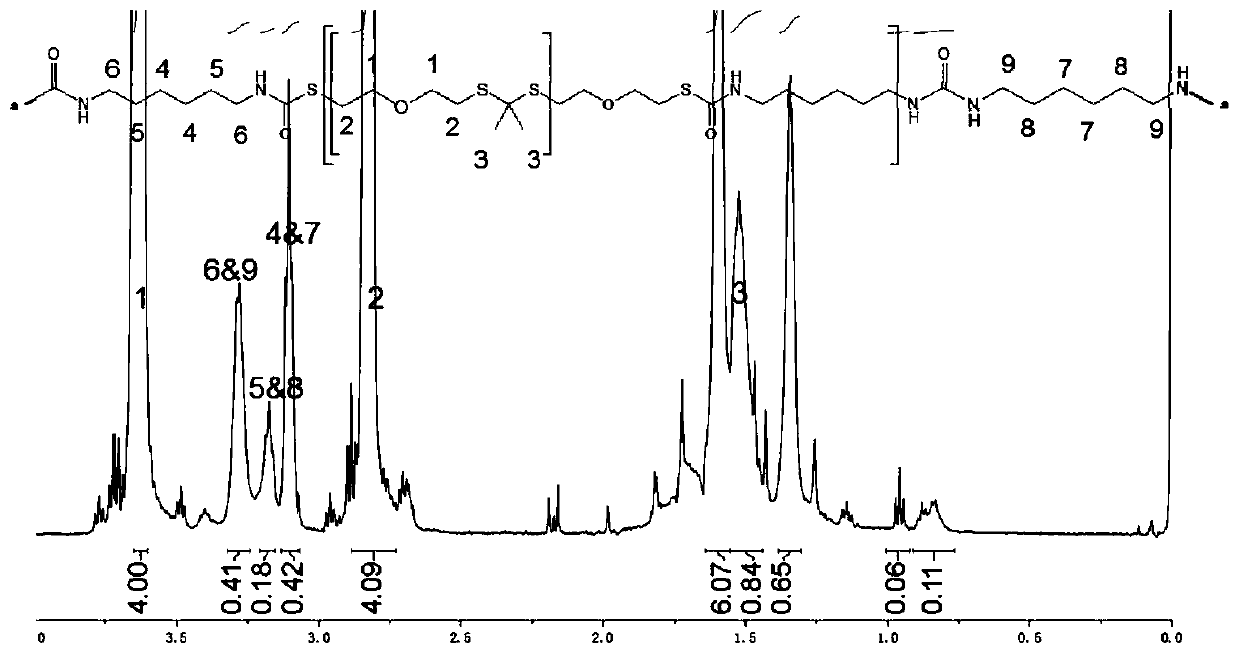
Polyurethane material containing polythioketal soft segment and capable of being degraded with active oxygen, and preparation method thereof
A technology of polyurethane material and polyketal thiol, which is applied in the field of polyurethane material degraded by active oxygen and its preparation, can solve the problems of non-degradation of polyurethane, tissue inflammatory response, slow process, etc., and achieve good biocompatibility effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Step 1: Under nitrogen protection, stir 300ml of anhydrous acetonitrile, 30g of bis(2-mercaptoethyl)ether, and 0.56g of p-toluenesulfonic acid evenly, heat to reflux, and dropwise add 18.76g of 2,2- Dimethoxypropane, continue to react for 16h after the dropwise addition. After the solvent was removed by rotary evaporation, it was precipitated in ethanol three times and dried to obtain the polyketal thioketal with double-terminated mercapto groups. Its molecular weight and H NMR spectrum are as follows: figure 2 As shown, the results indicated that the polyketalthioketal was successfully synthesized.
[0030] The second step: add 1 g of the polyketal thioketal prepared in the first step to the reactor and completely remove water, add 5 ml of N,N-dimethylformamide under nitrogen protection, add 121 mg of hexamethylene diisocyanate, and then separately Add 50 ul of catalyst triethylamine and 10 ul of dibutyltin dilaurate, and heat at 80°C under nitrogen protection for 4...
Embodiment 2
[0035] Step 1: Under nitrogen protection, stir 100ml of anhydrous acetonitrile, 30g of bis(2-mercaptoethyl)ether, and 0.56g of p-toluenesulfonic acid evenly, heat to reflux, and dropwise add 18.76g of 2,2- Dimethoxypropane, continue to react for 16h after the dropwise addition. After the solvent was removed by rotary evaporation, it was precipitated in ethanol three times and dried to obtain the polyketal thioketal with double-terminated mercapto groups.
[0036] Step 2: Add 0.5 g of the polyketal thioketal prepared in the first step to the reactor, then add 0.75 g of polyethylene glycol (molecular weight 2000), completely remove water, and add 5 ml of N,N-dimethyl 221 mg of 4,4'-dicyclohexylmethane diisocyanate was added, and then 50 ul of catalyst triethylamine and 10 ul of dibutyltin dilaurate were added, and the reaction was pre-polymerized by heating at 80°C for 4 hours under the protection of nitrogen.
[0037]The third step: the temperature is lowered to 70° C., 12.6 m...
Embodiment 3
[0041] Step 1: Under nitrogen protection, stir 100ml of anhydrous acetonitrile, 30g of bis(2-mercaptoethyl)ether, and 0.56g of p-toluenesulfonic acid evenly, heat to reflux, and dropwise add 18.76g of 2,2- Dimethoxypropane, continue to react for 16h after the dropwise addition. After the solvent was removed by rotary evaporation, it was precipitated in ethanol three times and dried to obtain the polyketal thioketal with double-terminated mercapto groups.
[0042] The second step: add 0.5g of polyketal thioketal prepared in the first step to the reactor, then add 0.5g of polyethylene glycol, completely remove water, add 5ml of N,N-dimethylformamide under nitrogen protection, Add 181.4 mg of 4,4'-dicyclohexylmethane diisocyanate, and then add catalyst triethylamine 50 ul and dibutyltin dilaurate 10 ul respectively, and heat reaction at 80° C. under nitrogen protection for 4 hours for pre-polymerization.
[0043] The third step: lower the temperature to 70° C., add 10.4 mg of 1,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com