Preparation method of atosiban acetate impurities
A technology for atosiban acetate and impurities, which is applied in the field of preparation of atosiban acetate impurities, and can solve problems such as toxicity, adverse reactions, and allergic reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
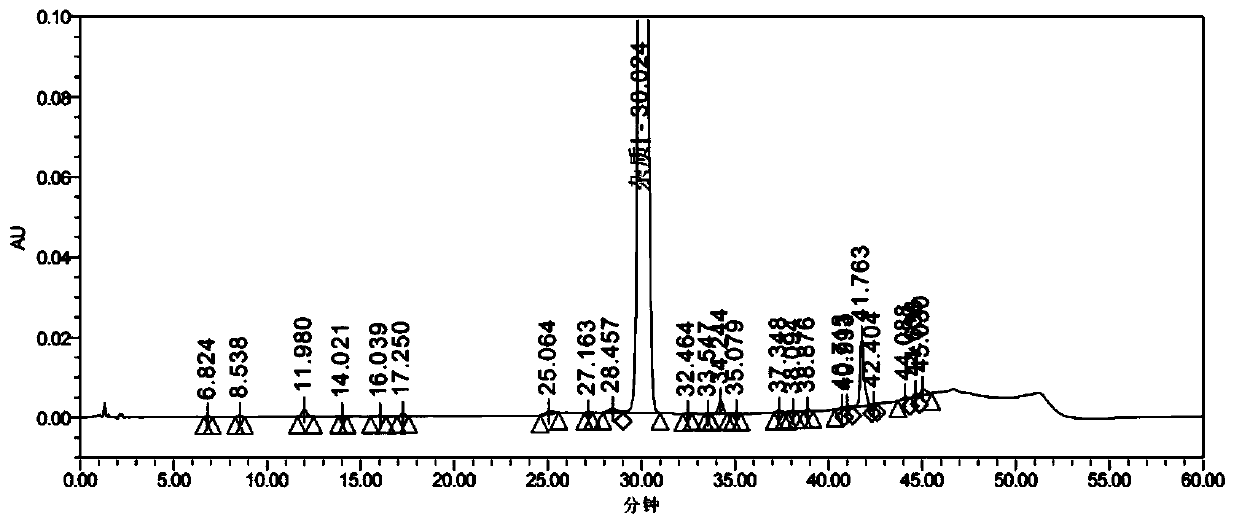
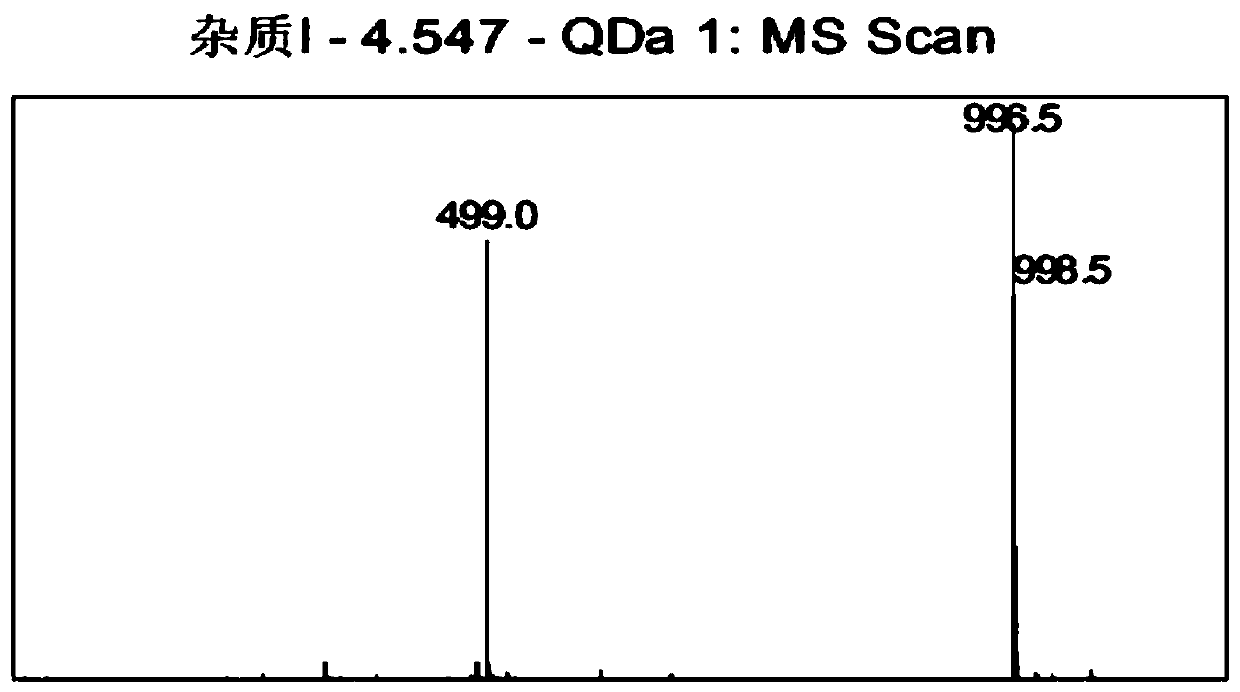
[0067] Embodiment 1: Impurity I [Asp 5 ,Gly 9 -OH] Preparation
[0068] Weigh 12.5g of Wang resin (10mmol, substitution value: 0.80mmol / g), add to the peptide synthesis reaction column, add 90ml of DCM to swell for 30min, and drain. Wash twice with DMF and drain. Weigh 8.92g Fmoc-Gly-OH (3.0eq), 5.40g HOBt (4.0eq), 0.37g DMAP (0.3eq) and dissolve in 50ml DMF, add 6.2ml DIC (4.0eq) at 0℃~10℃ , activated for 5 minutes, added to the synthesis reaction column, stirred and reacted at 30°C±3°C for 3-4h. Drained and washed 3 times with DMF. Add 20% PIP / DMF to remove Fmoc protection twice (the time is 5min+10min respectively), and wash with DMF 6 times.
[0069]Weigh 13.63g Fmoc-Orn(Boc)-OH (3.0eq), 5.40g HOBt (4.0eq) and dissolve in 50ml DMF, add 6.2ml DIC (4.0eq) at 0℃~10℃, activate for 5min, add In the synthesis reaction column, stir and react at 30°C±3°C for 2-3 hours, and the ninhydrin test is negative. Drained and washed 3 times with DMF. Add 20% PIP / DMF to remove Fmoc p...
Embodiment 2
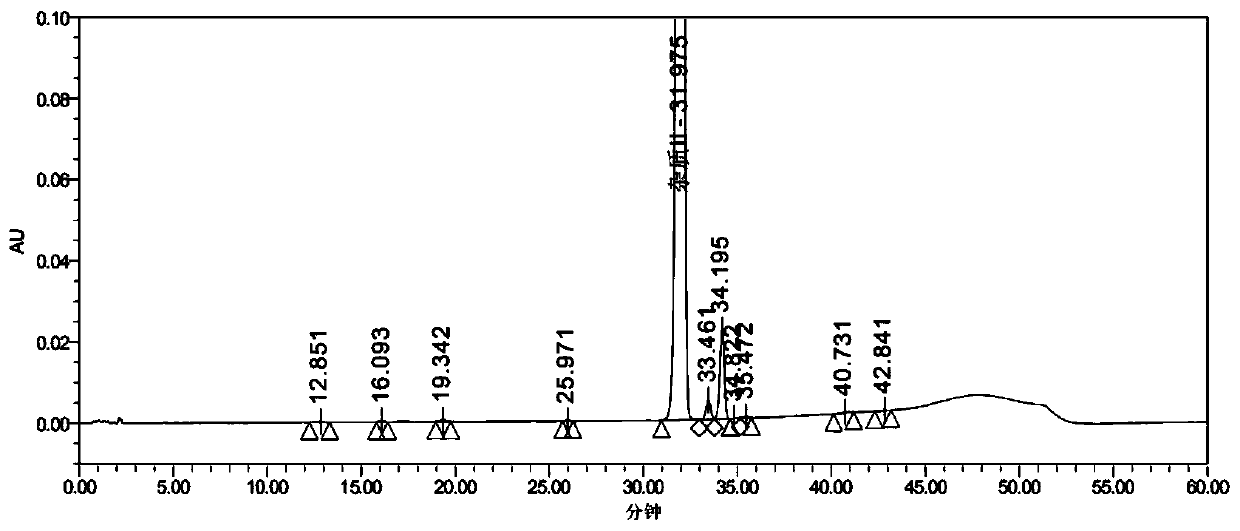
[0073] Embodiment 2: Impurity II [Orn(Ac) 8 ] preparation
[0074] Weigh 10.6g Rink Amide AM resin (10mmol, substitution value 0.95mmol / g), add to the peptide synthesis reaction column, add 70ml DCM to swell for 30min, and drain. Wash twice with DMF and drain. Add 20% PIP / DMF to remove Fmoc protection twice (the time is 5min+10min respectively), and wash with DMF 6 times.
[0075] Weigh 8.92g Fmoc-Gly-OH (3.0eq), 5.40g HOBt (4.0eq) and dissolve in 40ml DMF, add 6.2ml DIC (4.0eq) at 0℃~10℃, activate for 5min, add to the synthesis reaction column During the reaction at 30°C±3°C for 2 to 3 hours with stirring, the ninhydrin test was negative. Drained and washed 3 times with DMF. Add 20% PIP / DMF to remove Fmoc protection twice (the time is 5min+10min respectively), and wash with DMF 6 times.
[0076] Repeat the above steps and sequentially couple Fmoc-Orn(Ac)-OH, Fmoc-Pro-OH, Fmoc-Cys(Trt)-OH, Fmoc-Asp(OtBu)-OH, Fmoc-Thr(tBu)-OH, Fmoc -Ile-OH, Fmoc-D-Tyr(Et)-OH, Mpa(Trt)-OH....
Embodiment 3
[0079] Example 3: Preparation of Impurity III [Dimer 1]
[0080] Weigh 10.6g Rink Amide AM resin (10mmol, substitution value 0.95mmol / g), add to the peptide synthesis reaction column, add 70ml DCM to swell for 30min, and drain. Wash twice with DMF and drain. Add 20% PIP / DMF to remove Fmoc protection twice (the time is 5min+10min respectively), and wash with DMF 6 times.
[0081] Weigh 8.92g Fmoc-Gly-OH (3.0eq), 5.40g HOBt (4.0eq) and dissolve in 40ml DMF, add 6.2ml DIC (4.0eq) at 0℃~10℃, activate for 5min, add to the synthesis reaction column During the reaction at 30°C±3°C for 2 to 3 hours with stirring, the ninhydrin test was negative. Drained and washed 3 times with DMF. Add 20% PIP / DMF to remove Fmoc protection twice (the time is 5min+10min respectively), and wash with DMF 6 times.
[0082] Repeat the above steps and sequentially couple Fmoc-Orn(Boc)-OH, Fmoc-Pro-OH, Fmoc-Cys(Trt)-OH, Fmoc-Asp(OtBu)-OH, Fmoc-Thr(tBu)-OH, Fmoc -Ile-OH, Fmoc-D-Tyr(Et)-OH, Mpa(Acm)-OH. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com