Reagent kit for detecting streptococcus pyogenes
A technology of Streptococcus pyogenes and kits, which is applied in the determination/testing of microorganisms, biochemical equipment and methods, etc., can solve the problems of high cost, delay in disease treatment, lack of specific primers, etc., and achieve high specificity and equipment requirements low effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
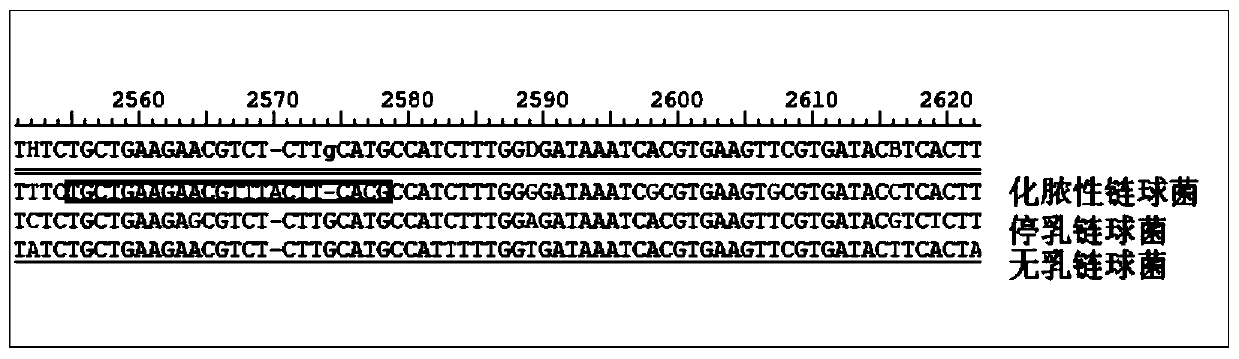
[0014] Embodiment 1: Streptococcus rpoB gene sequence alignment
[0015] Such as figure 1 As shown, in the rpoB gene sequence of Streptococcus pyogenes, the 5' end of the guide RNA sequence (selected part) of the present invention is the PAM structure of TTTN. The guide RNA sequence does not have a completely consistent sequence in Streptococcus dysgalactiae and Streptococcus agalactiae, and there are several base differences. Moreover, there is no PAM structure of TTTN at the 5' end of the homologous sequence in Streptococcus dysgalactiae and Streptococcus agalactiae, so using this guide RNA, the Cas12a protein cannot be targeted to Streptococcus dysgalactiae and Streptococcus agalactiae, so , when testing, only Streptococcus pyogenes can show a positive reaction, and the rest of Streptococcus pyogenes are negative, which can be used for the specific detection of Streptococcus pyogenes.
Embodiment 2
[0016] Embodiment 2: The guide RNA specificity determination of the present invention, the steps are as follows:
[0017] (1) Prepare Streptococcus pyogenes, Streptococcus dysgalactiae, Streptococcus agalactiae standard strains;
[0018] (2) Take 1ml of the sample to be tested, heat it at 98 degrees Celsius for 5min, and draw 1μl as the test sample;
[0019] (3) Prepare the reaction system, the reaction system is 25 μl, including 1 μl detection sample, 14.75 μl hydrated TwistAmp basickit reaction drying ball (TwistDx company), 0.9 μl 10mM RPA-F (sequence is SEQ No.2) and RPA-R ( Sequence is SEQNo.3), 0.375 μl Ribonuclease Inhibitor (Takara company), 3.5 μl buffer2.1 (NEB company), 1000nM guide RNA (sequence is SEQ No.1), 250nM Cas12a (NEB company), 200nM single-stranded DNA probe Needle (Shanghai Sangong), add water to make up to 25μl;
[0020] (4) React at a constant temperature of 37 degrees Celsius for 30 minutes;
[0021] (5) After the reaction, place the PCR tube under...
Embodiment 3
[0023] Embodiment 3: the detection method based on guide RNA described in the present invention and traditional PCR method specificity and experimental time-consuming comparison
[0024] 1. based on the detection method of guide RNA described in the present invention, the steps are as follows:
[0025] (1) Prepare Streptococcus pyogenes, Streptococcus dysgalactiae, Streptococcus agalactiae standard strains;
[0026] (2) Take 1ml of the sample to be tested, heat it at 98 degrees Celsius for 5min, and draw 1μl as the test sample;
[0027] (3) Prepare the reaction system, the reaction system is 25 μl, including 1 μl detection sample, 14.75 μl hydrated TwistAmp basickit reaction drying ball (TwistDx company), 0.9 μl 10mM RPA-F (sequence is SEQ No.2) and RPA-R ( Sequence is SEQNo.3), 0.375 μl Ribonuclease Inhibitor (Takara company), 3.5 μl buffer2.1 (NEB company), 1000nM guide RNA (sequence is SEQ No.1), 250nM Cas12a (NEB company), 200nM single-stranded DNA probe Needle (Shanghai...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com