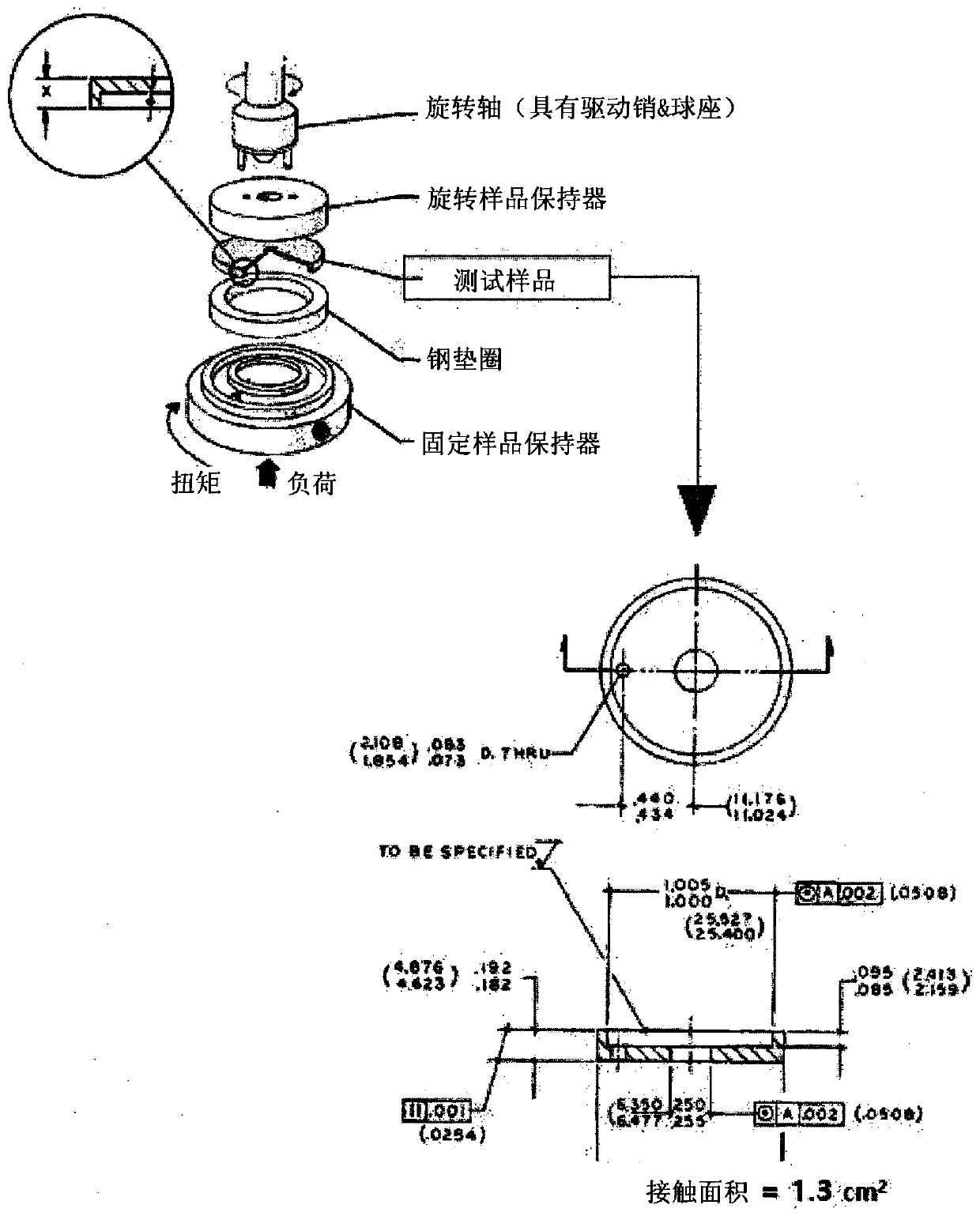
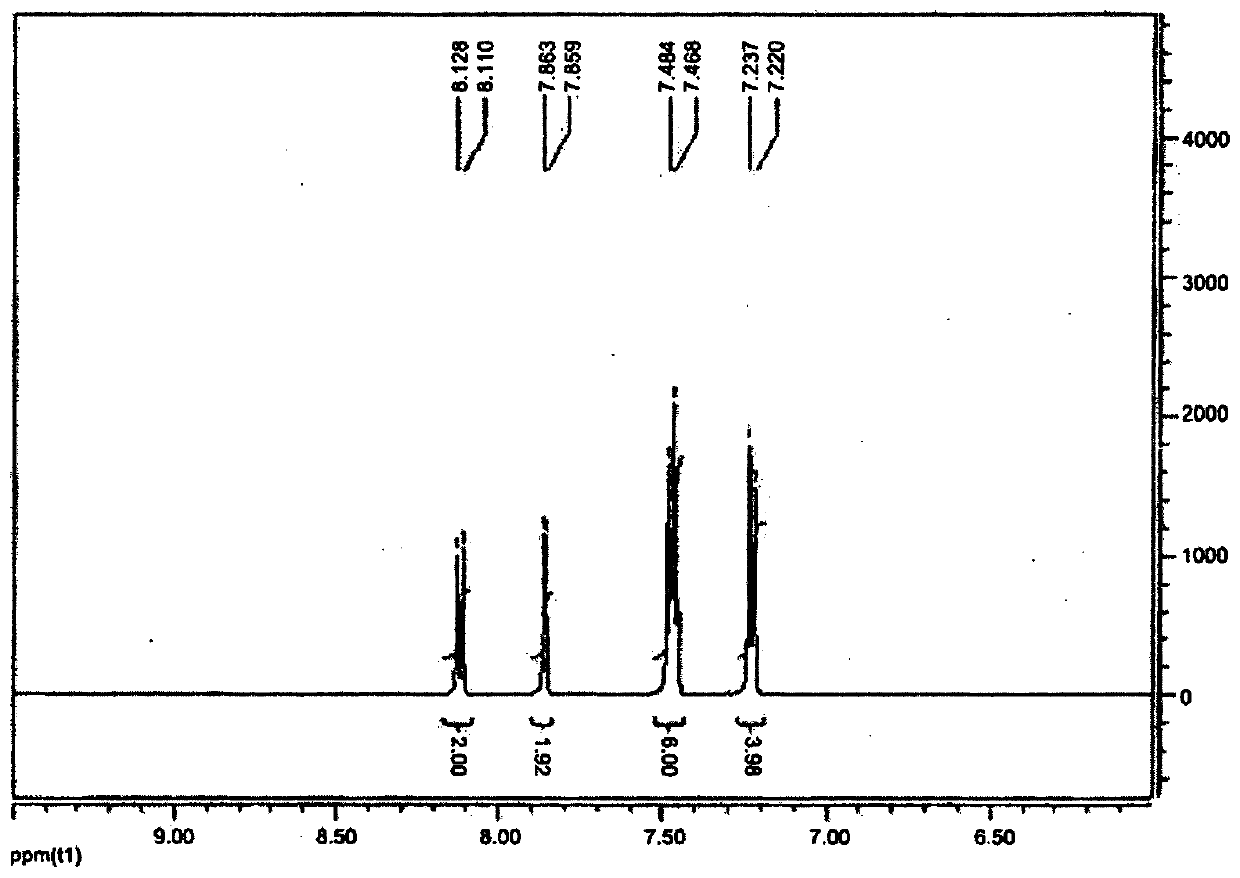
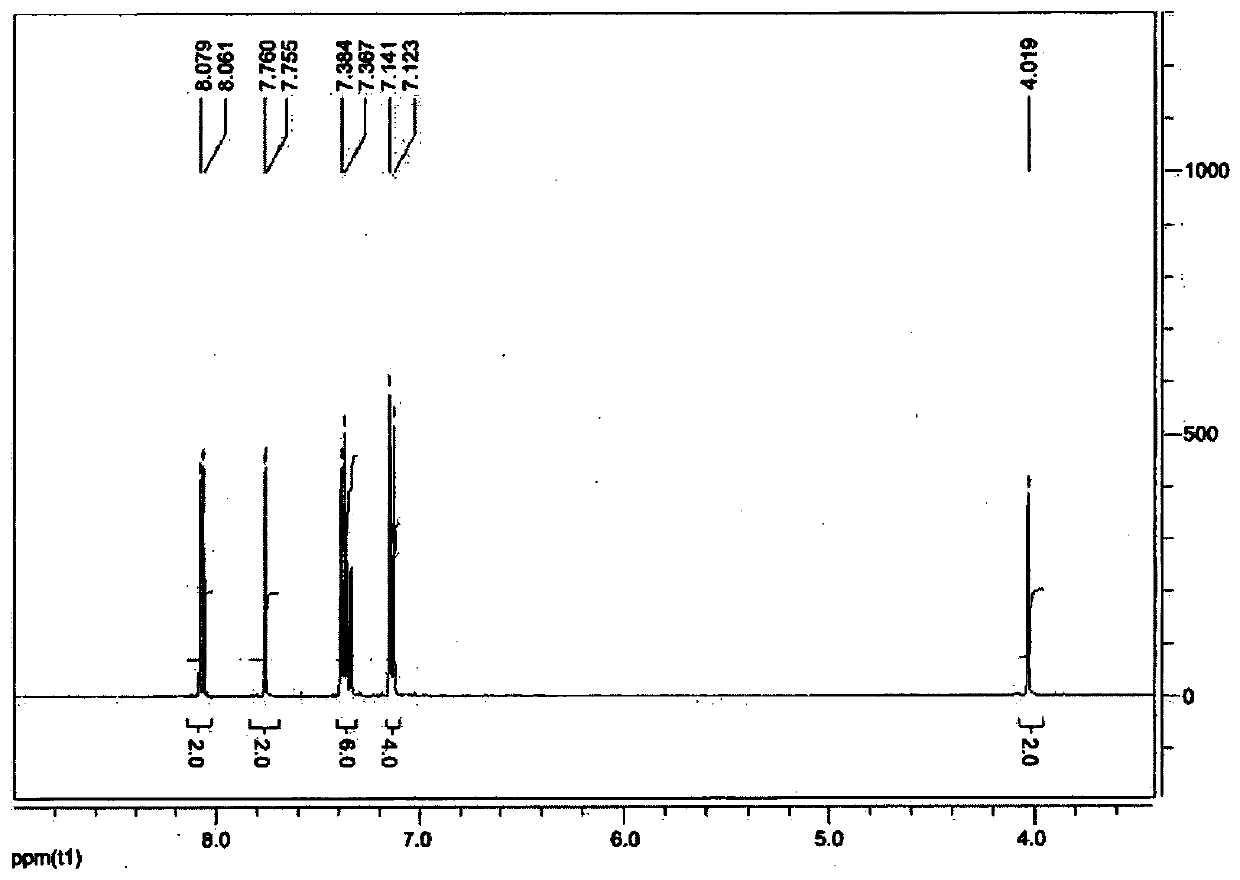
Low-friction polymerizable composition
A polymer composition and low-friction technology, applied in lubricating compositions, base materials, petroleum industry, etc., can solve the problems of low processability and productivity, low heat resistance, high cost, etc., and achieve high heat resistance, Excellent processability, durability and reliability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0247] Preparation example 1. Synthesis of phthalonitrile compound (PN1)
[0248] The compound (PN1) of the following chemical formula A1 was synthesized as follows.
[0249] 32.7 g of a compound of the following chemical formula A2 and 120 g of DMF (dimethylformamide) were introduced into a 3-neck round bottom flask, and stirred at room temperature to dissolve. Subsequently, 51.9 g of the compound of the following chemical formula A3 was added, and 50 g of DMF was added, followed by stirring to dissolve. Subsequently, 62.2 g of potassium carbonate was introduced together with 50 g of DMF, and the temperature was raised to 85° C. while stirring. After reacting for about 5 hours, the solution was cooled to room temperature.
[0250] The cooled reaction solution was poured into a 0.2N aqueous hydrochloric acid solution to neutralize and precipitate it, and after filtration, it was washed with water. Thereafter, the filtered reactant was dried in a vacuum oven at 100° C. for...
preparation example 2
[0257] Preparation example 2. Synthesis of phthalonitrile compound (PN2)
[0258] The compound (PN2) of the following chemical formula A4 was synthesized as follows.
[0259] 28.0 g of 4,4'-bis(hydroxyphenyl)methane and 150 mL of DMF (dimethylformamide) were introduced into a 500 mL 3-necked round bottom flask, and stirred at room temperature to dissolve. Subsequently, 48.5 g of 4-nitrophthalonitrile was added, and 50 g of DMF was added, followed by stirring to dissolve. Subsequently, 58.1 g of potassium carbonate was introduced together with 50 g of DMF, and the temperature was raised to 85° C. while stirring. After reacting for about 5 hours, the solution was cooled to room temperature.
[0260] The cooled reaction solution was poured into a 0.2N aqueous hydrochloric acid solution to neutralize and precipitate it, and after filtration, it was washed with water. Thereafter, the filtered reactant was dried in a vacuum oven at 100° C. for one day, so that water and remaini...
preparation example 3
[0263] Preparation example 3. Synthesis of phthalonitrile compound (PN3)
[0264] The compound (PN3) of the following chemical formula A5 was synthesized as follows.
[0265] 160 g of a compound of the following chemical formula A6 and 200 g of DMF (dimethylformamide) were introduced into a 3-necked round bottom flask, and stirred at room temperature to dissolve. Subsequently, 52 g of the compound of the above chemical formula A3 was added, and 200 g of DMF was added, followed by stirring to dissolve. Subsequently, 62.2 g of potassium carbonate was introduced together with 100 g of DMF, and the temperature was raised to 85° C. while stirring. After reacting for about 5 hours, the solution was cooled to room temperature.
[0266] The cooled reaction solution was poured into 0.2N aqueous hydrochloric acid solution. Chloroform was added to the mixed solution to extract the product, and the extracted product was washed with water. Chloroform and the reaction solution DMF (di...
PUM
| Property | Measurement | Unit |
|---|---|---|
| decomposition temperature | aaaaa | aaaaa |
| friction coefficient | aaaaa | aaaaa |
| friction coefficient | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com