Synthesis process of paliperidone palmitate
A technology for paliperidone and a synthesis process, which is applied in the field of synthesis technology of paliperidone palmitate, can solve the problems of waste of resources, low production efficiency, large equipment damage, etc., and achieves low cost, controllable process, and cleanliness of impurities. control effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
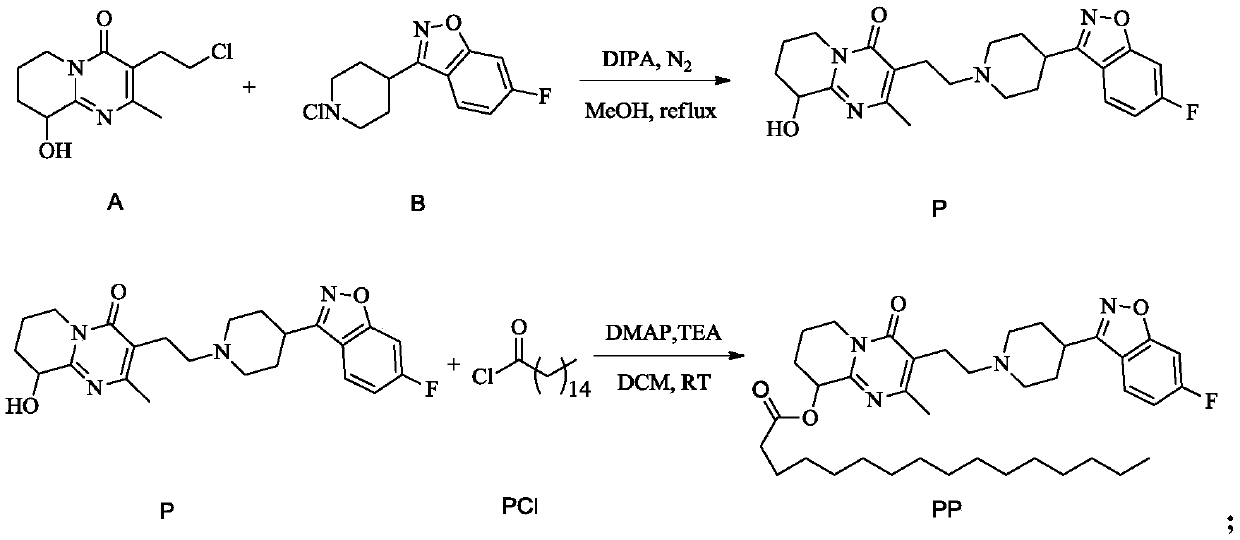
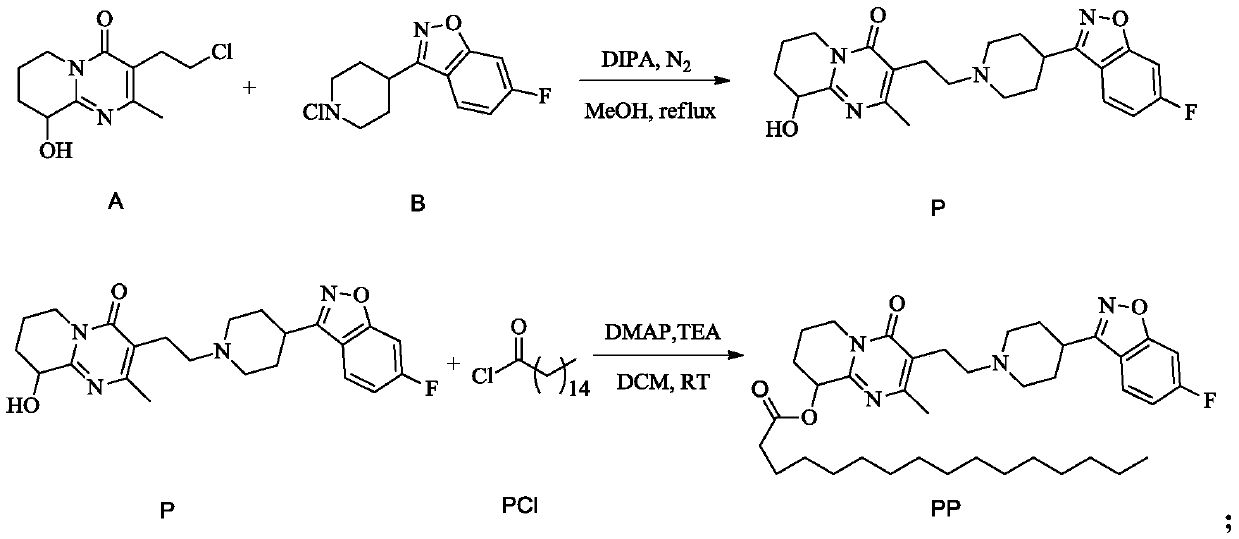
[0036] The synthesis of embodiment 1 paliperidone palmitate
[0037] (1) Synthesis of P:
[0038] a. Add compound A, compound B, DIPA and methanol into the flask, wherein the molar ratio of A:B:DIPA is 1:1:2.1; stir well, use N 2 Protection, start to heat up; reflux reaction for 11h, naturally cool down to 28°C after the reaction to crystallize;
[0039] b. Suction filter the crystal obtained in step a to obtain a filter cake; then the filter cake is beaten and washed with an aqueous solution of methanol with a mass concentration of 45% for 0.5 h; vacuum dried at 50° C. for 7 h to obtain compound P and detected; the yield is about 83.5% .
[0040] (2) Refining of P: feed intake, add the P crude product that step (1) prepares in the flask, then add ethanol and water, wherein the ethanol amount that every 100g P crude product adds is 1.1L by mass volume ratio, the addition of water The volume is 1.4L; in N 2 Heat up under protection until liquid reflux occurs; react until th...
Embodiment 2
[0051] The synthesis of embodiment 2 paliperidone palmitate
[0052] (1) Synthesis of P:
[0053] a. Add compound A, compound B, DIPA and methanol into the flask, wherein the molar ratio of A:B:DIPA is 1:1.2:2.3; stir well, use N 2 Protection, start to heat up; reflux reaction for 13h, naturally cool down to 32°C to crystallize after the reaction is over;
[0054] b. Suction filter the crystal obtained in step a to obtain a filter cake; then the filter cake is beaten and washed with an aqueous solution of methanol with a mass concentration of 55% for 1.5 hours; vacuum dried at 70° C. for 9 hours to obtain compound P and detected; the yield is about 85% .
[0055] (2) Refining of P: feed intake, add the P crude product that step (1) prepares in the flask, then add ethanol and water, wherein the ethanol amount that every 100g P crude product adds is 1.2L by mass volume ratio, the addition of water The volume is 1.6L; in N 2 Heat up under protection until liquid reflux occurs...
Embodiment 3
[0066] The synthesis of embodiment 3 paliperidone palmitate
[0067] (1) Synthesis of P:
[0068] a. Add compound A, compound B, DIPA and methanol into the flask, wherein the molar ratio of A:B:DIPA is 1:1.1:2.2; stir well, use N 2 Protect, start to heat up; reflux reaction for 12 hours, naturally cool down to 30°C after the reaction to crystallize; the yield is about 89.5%;
[0069] b. Suction filter the crystals obtained in step a to obtain a filter cake; then the filter cake is beaten and washed with an aqueous solution of methanol with a mass concentration of 50% for 1 h; vacuum-dried at 60° C. for 8 h to obtain compound P and detected.
[0070] (2) Refining of P: feed intake, add the P crude product that step (1) prepares in the flask, then add ethanol and water, wherein the ethanol amount that every 100g P crude product adds is 1.2L by mass volume ratio, the addition of water The volume is 1.5L; in N 2 Heat up under protection until liquid reflux occurs; react until t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com