Tetrahydronaphthalene and tetrahydroisoquinoline derivatives as estrogen receptor degraders
A technology of solvates and stereoisomers, applied in the field of tetrahydronaphthalene and tetrahydroisoquinoline derivatives as estrogen receptor degraders, can solve problems such as increase in MDM2
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[2204] pass 1 All synthesized compounds were characterized by H-NMR and analyzed for purity by LC / MS at wavelengths of 214 and 254 nM with UV detection. The purity of each compound in Table 1 and Table 2 was over 90%. The molecular weights observed from LC / MS are listed in Table 1 (see Figure 5 ) and Table 2 (see Figure 6 ) as [M+H] + listed. The synthetic methods used to prepare the respective compounds are listed in Table 1 and Table 2. Some molecules in Tables 1 and 2 are available as salts such as hydrochloride, acetate, formate or triflate. Only the structure of the neutral form of each compound is listed. 代表性化合物的1 H-NMR is listed in Table 3 (see Figure 7 ). Although the chemical names listed in Table 3 are for the neutral forms of the exemplary compounds, the corresponding 1 H-NMR data include both neutral and salt forms.
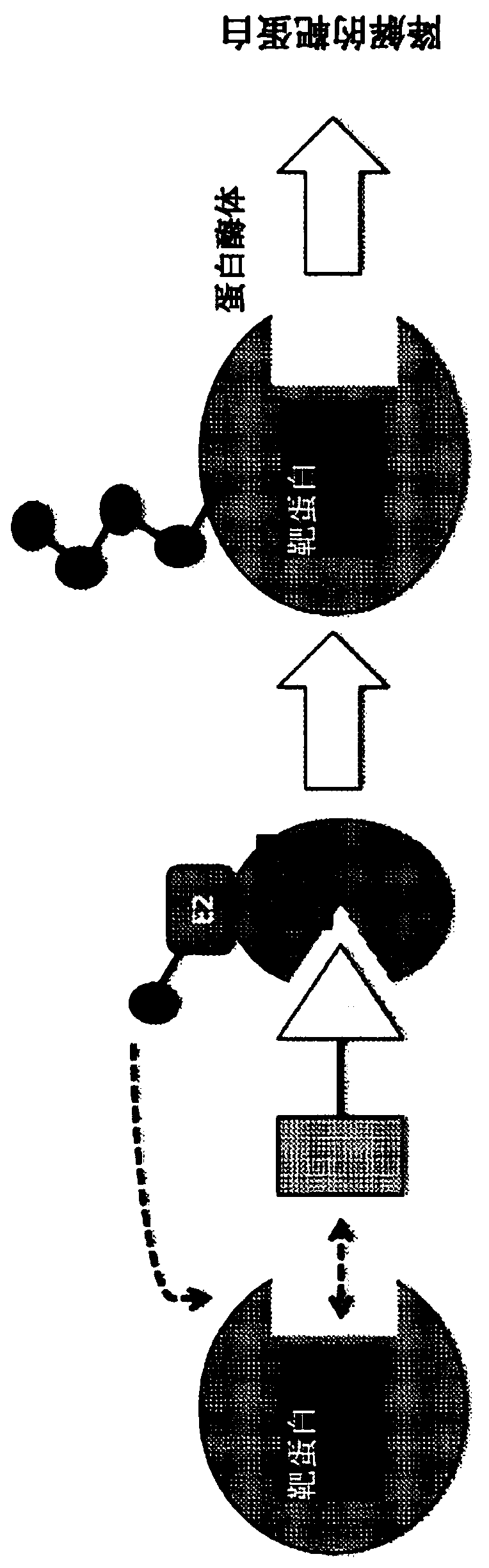
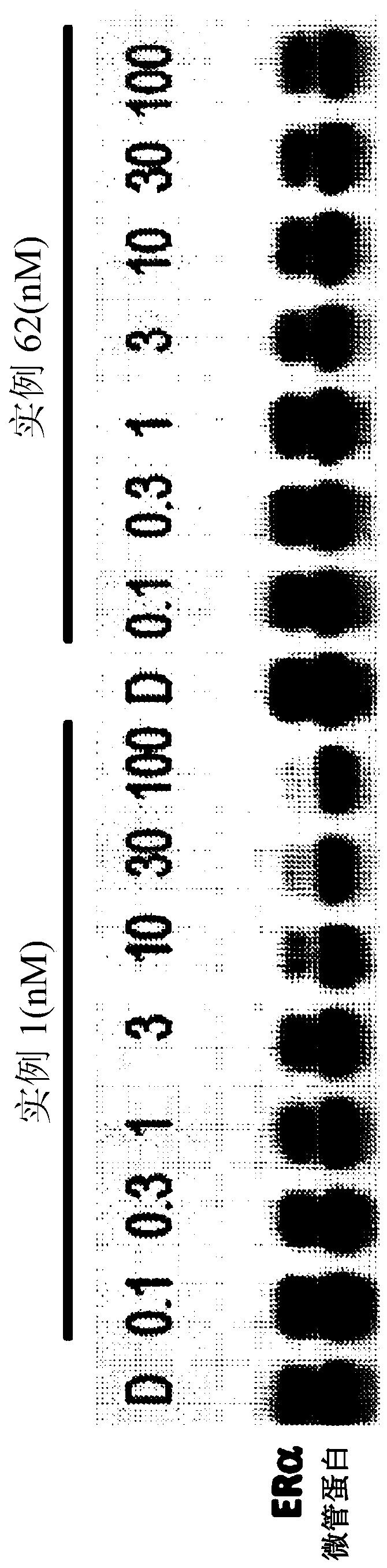
[2205] All synthetic chimeric molecules were evaluated for target engagement in T47D cells using a commercial kit for the ERE luciferase...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com