Medicine composition containing interleukin-38 recombinant protein and application of medicine composition
A technology of interleukin and recombinant protein, applied in the field of pharmaceutical compositions containing interleukin-38 recombinant protein, can solve the problem of no treatment means
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
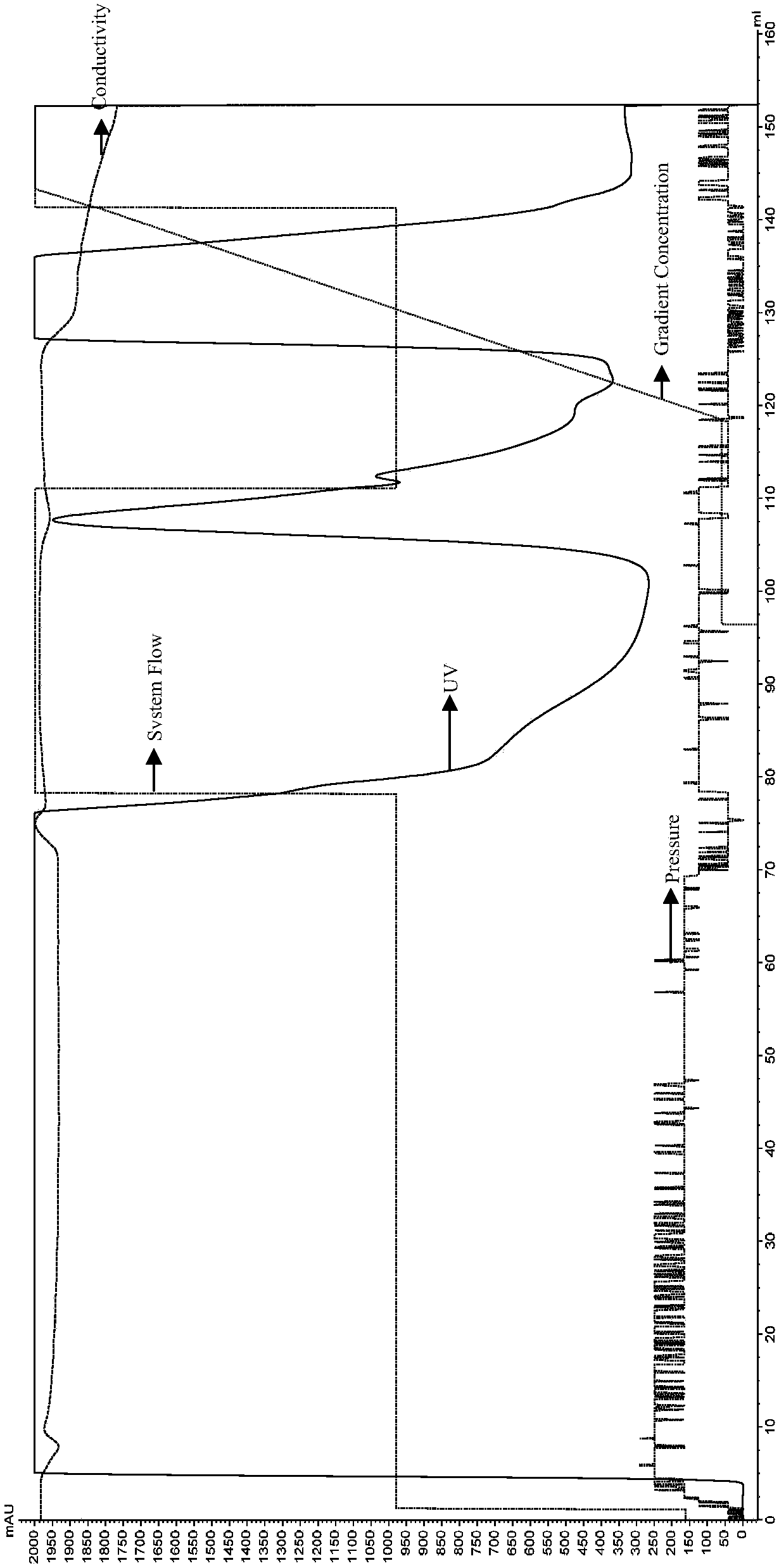
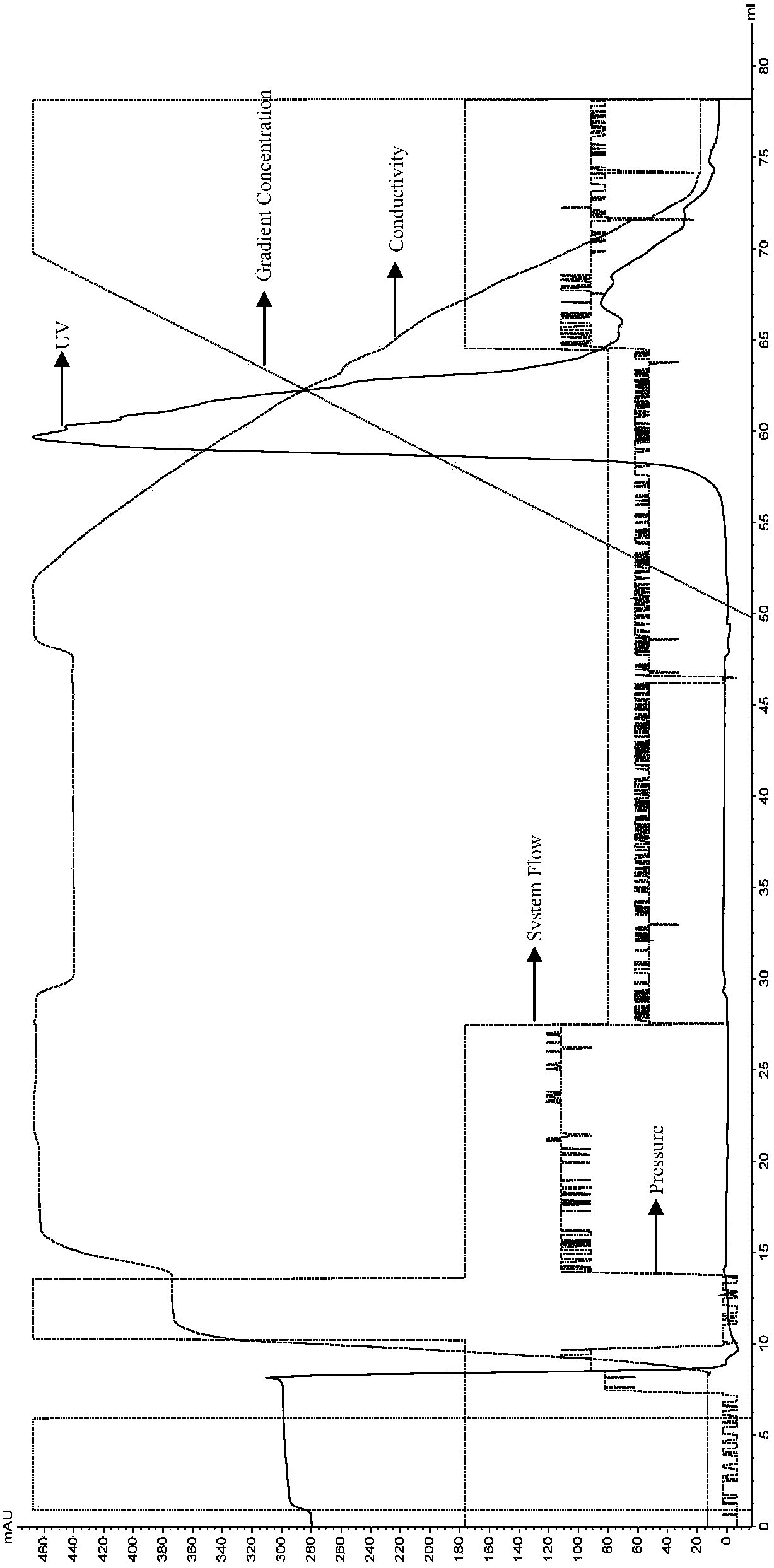
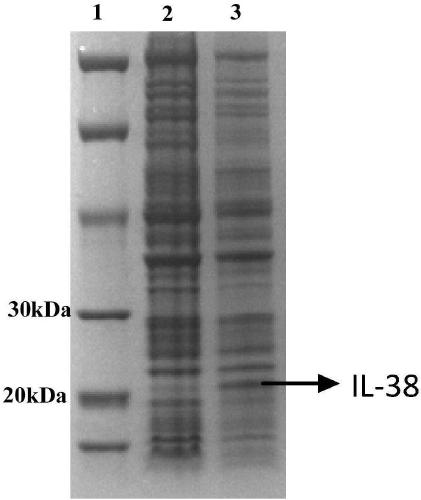
[0056] Embodiment 1: Expression of IL-38 recombinant protein
[0057] According to Sequence 1 and Sequence 2 in the sequence table, the IL-38 recombinant protein gene fragment was synthesized and connected into the pET-24 expression vector (Suzhou Synbio Biotechnology Co., Ltd.), and the recombinant plasmid was transformed into BL-21 competent strain (Bio -Rad). Wherein, Sequence 2 is the DNA sequence corresponding to the amino acid sequence shown in Sequence 1.
[0058] Inoculate the transformed BL-21 positive clone into 50mL of LB medium, cultivate at 37°C and 220rpm until the OD600 reaches about 0.6, then inoculate 2 bottles of 500ml LB medium with 1% inoculum, and continue to cultivate until the OD600 reaches 0.6, then add IPTG (final concentration 0.5mM) at 30°C, 220rpm to induce expression overnight.
[0059] The total volume of 1 L of BL21 bacterial solution induced to express overnight was centrifuged (8000×g, 15 min, 4° C.), and the bacterial cells were collected....
Embodiment 2
[0076] Embodiment 2: Enzyme-linked immunosorbent assay (ELISA) detects IL-38 protein and IL-36 protein and IL-36R-Fc affinity
[0077] Experimental steps:
[0078] (1) Coat the ELISA plate with IL-38 protein and IL-36 protein at a concentration of 2 μg / mL in a volume of 100 μL, and overnight at 4°C;
[0079] (2) Wash the plate 5 times with 0.1% PBST;
[0080] (3) Block the ELISA plate with 5% BSA solution;
[0081] (4) Wash the plate 5 times with 0.1% PBST;
[0082] (5) Dilute the IL-36R-Fc solution with an initial concentration of 50 μg / mL to 16.7 μg / mL, 5.56 μg / mL, 1.85 μg / mL, 0.62 μg / mL, 0.21 μg / mL according to a 1:3 concentration gradient , 0.07μg / mL, each gradient concentration of IL-36R-Fc solution was added to the ELISA plate, the final volume was 50μL, and incubated at 37°C for 1.5 hours;
[0083](6) Wash the plate 5 times with 0.1% PBST;
[0084] (7) Dilute the HRP-labeled anti-human Fc polyclonal antibody at 1:2000, add 100 μL per well to the ELISA plate, an...
Embodiment 3
[0088] Example 3: ELISA method to detect the competitive binding of IL-38 protein and IL-36 protein to IL-36R-Fc
[0089] Experimental steps:
[0090] (1) Coat the ELISA plate with IL-36 at a concentration of 2 μg / mL in a volume of 100 μL, overnight at 4°C;
[0091] (2) Wash the plate 5 times with 0.1% PBST;
[0092] (3) Block the ELISA plate with 5% BSA solution;
[0093] (4) Wash the plate 5 times with 0.1% PBST;
[0094] (5) Dilute the IL-38 protein solution with an initial concentration of 100 μg / mL to 10 μg / mL, 1 μg / mL, and 0.1 μg / mL in a concentration gradient of 1:10, and add IL-38 to each concentration gradient of IL-38 solution. The final concentrations of 36R-Fc and IL-36R-Fc were both 10 μg / mL, added to the ELISA plate with a final volume of 50 μl, and incubated at 37°C for 1.5 hours;
[0095] (6) Wash the plate 5 times with 0.1% PBST;
[0096] (7) Dilute the HRP-labeled anti-human Fc polyclonal antibody at 1:2000, add 100 μL per well to the ELISA plate, and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com